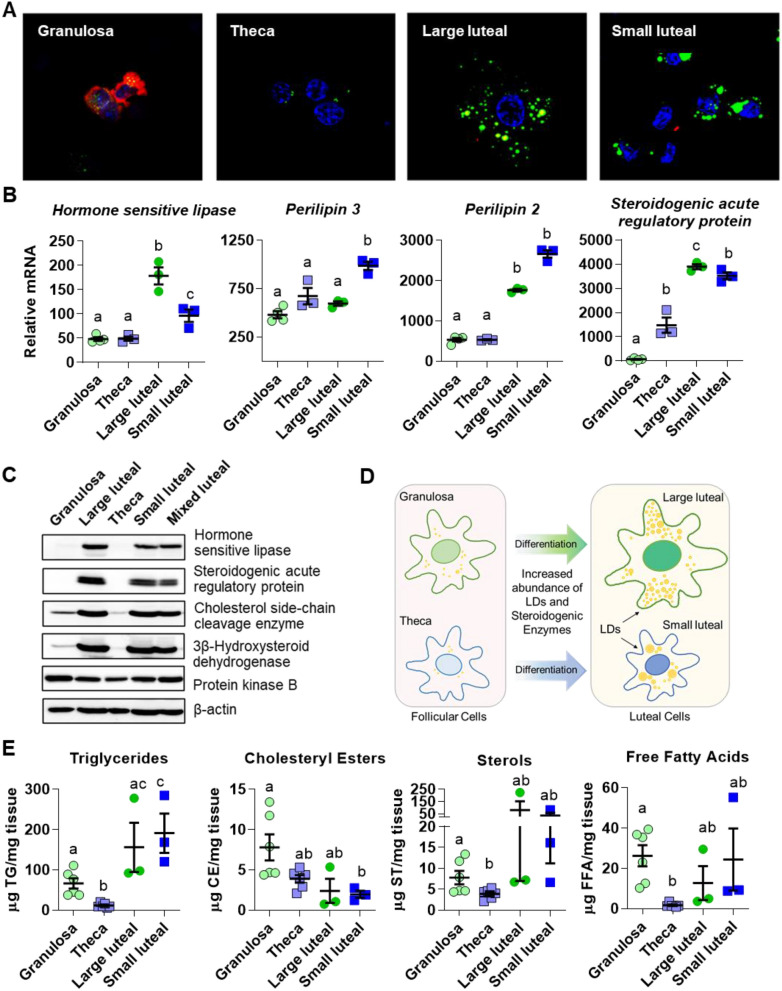
Figure 3.
Comparison of follicular and luteal LD properties. Follicular granulosa cells, theca cells, large luteal cells, and small luteal cells were isolated from bovine ovaries as described in the Methods. Panel (A) Confocal fluorescent image showing LD staining in freshly isolated bovine granulosa, theca, large luteal, and small luteal cells. LDs were stained with the neutral lipid dye BODIPY 493/503 (green), and cells were immuno-labeled using an aromatase antibody to specifically label granulosa cells (red), and the nuclei are counter-stained with 4′,6-diamidino-2-phenylindole (DAPI, blue). All images are equal magnification. Panel (B) Microarray analysis of mRNA abundance of LD-coat proteins: perilipin 2 and 3, cholesteryl esterase: hormone sensitive lipase, and steroidogenic marker: steroidogenic acute regulatory protein. Significance determined in (34). Means with different letters differ significantly between cell types (P < 0.05). Panel (C) Western blot of LD-associated hormone sensitive lipase and steroidogenic enzymes in follicular granulosa and theca cells in comparison to large luteal, small luteal, and mixed luteal cells. Panel (D) Model depicting changes in LD number and proteins associated with luteal steroidogenesis during follicular cell differentiation. Panel (E) High-performance thin layer chromatography analysis of freshly isolated granulosa (n = 6), theca (n = 6), large luteal (n = 3) and small luteal cells (n = 3). Means ± SEM overlay individual measurements, significance was determined using mixed effects model and Tukey’s multiple comparisons test (factor 1: cell type (unmatched), factor 2: lipid class (matched)) after log transformation of values. Means with different letters differ significantly between cell types (P < 0.05).