FIGURE 5.
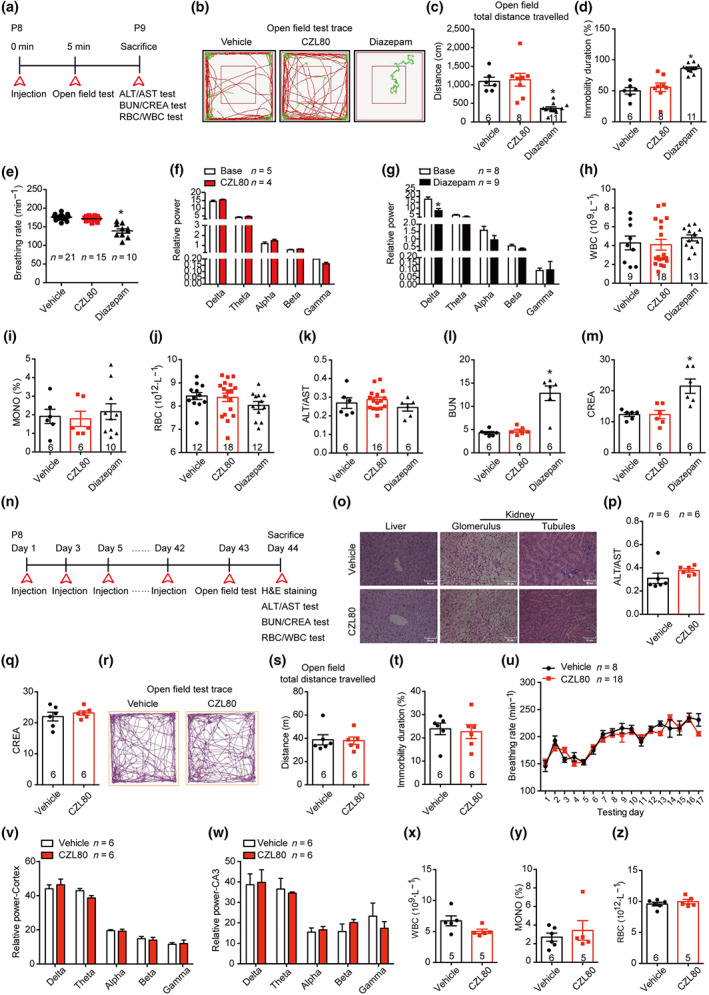
CZL80 has no obvious acute and chronic side effects. (a) Experimental paradigm of acute side effect test. (b–d) The representative tracts (b), total distance (c) and immobility duration (d) of vehicle, CZL80 (7.5 mg·kg−1, i.v.) or diazepam (0.3 mg·kg−1, i.v.)‐treated groups in open field tests. (e) The breathing rate (min−1) was tested in the vehicle, CZL80 or diazepam‐treated groups. (f, g) The EEG power in CZL80 (f) or diazepam‐treated group (g). (h–m) The WBC (h), MONO% (i), RBC (j), ALT/AST (k), BUN (l) and CREA (m) were tested in the vehicle, CZL80 and diazepam‐treated groups. (n) Experimental paradigm of chronic side effect test. (o) Representative images of morphological structure preservation of liver and kidney (Glomerulus and tubules) with HE staining in vehicle or CZL80‐treated groups, scale bar: 50 μm. (p, q) ALT/AST (p) and CREA (q) were tested in vehicle or CZL80‐treated groups. (r–t) Representative tracks (r), total distance (s) and immobility duration (t) in vehicle (2% DMSO in saline, qod for 6 weeks, i.v.) or CZL80 (7.5 mg·kg−1, qod for 6 weeks, i.v.)‐treated groups in the open field test. (u) The breathing rate was tested in vehicle or CZL80‐treated groups. (v, w) The EEG activity power of cortex (v) and hippocampal CA3 region (w) in vehicle or CZL80‐treated groups. (x–z) The WBC (x), MONO% (y) and RBC (z) were tested in vehicle or CZL80‐treated groups. Values are means ± SEM. *P < .05, significantly different from vehicle. One‐way ANOVA followed by Dunnett post hoc test was used for (c)–(e), (l) and (m). Unpaired t test was used for (g)
