Abstract
Coronaviruses such as MERS-CoV and SARS-CoV-2 infect the human respiratory tract and can cause severe pneumonia. Disease severity and outcomes are different for these two infections: the human mortality rate for MERS-CoV and SARS-CoV-2 is over 30% and less than 10%, respectively. Here, using microarray assay, we analyzed the global alterations in gene expression induced by MERS-CoV or SARS-CoV-2 infections in primary human pulmonary epithelial cells. Overall, the number of differentially expressed genes was higher in human lung cells infected with MERS-CoV than in cells with SARS-CoV-2. Out of 44,556 genes analyzed, 127 and 50 were differentially expressed in cells infected with MERS-CoV and SARS-CoV-2, respectively (> 2-fold increase, compared to uninfected cells). Of these, only eight genes, including the one coding for CXCL8, were similarly modulated (upregulated or downregulated) by the two coronaviruses. Importantly, these results were virus-specific and not conditioned by differences in viral load, and viral growth curves were similar in human lung cells infected with both viruses. Our results suggest that these distinct gene expression profiles, detected early after infection by these two coronaviruses, may help us understand the differences in clinical outcomes of MERS-CoV and SARS-CoV-2 infections.
Electronic supplementary material
The online version of this article (10.1007/s00705-020-04730-3) contains supplementary material, which is available to authorized users.
Introduction
Coronaviruses are enveloped, positive-sense single-stranded RNA viruses belonging to the family Coronaviridae [1]. Their genomes are approximately 30 kb in length, are polycistronic, and have a peculiar transcription mechanism that results in the production of a nested set of subgenomic mRNAs [1]. Their virions are mainly composed of four structural proteins: nucleocapsid (N), membrane (M), envelope (E), and spike (S) proteins, all of which are essential building blocks for virion formation [1]. The spike proteins are the most external structures of coronavirus particles, and they are responsible for attachment of the virus to target cells (via specific ligand-receptor interactions) and, consequently, for the initiation of infection [1].
Coronaviruses are recognized animal and human pathogens [2]. Up to the end of 2019, there were six coronaviruses known to cause respiratory disease in humans, with varying degrees of severity: human coronavirus (HCoV)-OC43, HCoV-229E, HCoV-NL63, HCoV-HKU1, severe acute respiratory syndrome coronavirus (SARS-CoV), and Middle East respiratory syndrome coronavirus (MERS-CoV) [1, 3]. MERS-CoV emerged in the Saudi Arabia in 2012 and has since spread to 26 other countries [4]. Infection is known to cause a wide range of manifestations, varying from asymptomatic to acute respiratory distress syndrome, which can evolve to circulatory collapse, multiorgan failure, and death [5]. According to the World Health Organizations (WHO), as of the end of November 2019, 2494 laboratory-confirmed human cases have been reported, 858 of which were fatal (34.4% case-fatality rate).
In December 2019, a cluster of cases of atypical pneumonia of unknown etiology in Wuhan, China, attracted global attention [6–8]. A novel coronavirus was isolated from these patients, and named SARS-CoV-2. Infection with this new virus also has a wide range of clinical manifestations, from asymptomatic, to mild fever with dry cough, to severe pneumonia [9].
In this study, we analyzed the global gene expression of human pulmonary epithelial cells infected with MERS-CoV or SARS-CoV-2 by microarray to understand the pulmonary pathological consequences of infection by these two coronaviruses.
Materials and methods
Viruses and cells
The SARS-CoV-2 strain hCoV-19/Korea/CNUHV03/2020 was isolated in our laboratory from human clinical samples collected at the Chungnam National University Hospital (Daejeon, South Korea). Vero cells, cultured in minimal essential medium (MEM) supplemented with 2% fetal bovine serum (FBS), were used in the isolation protocol. The MERS-CoV strain EMC2012 was kindly provided by Dr. Bart Haagmans and Dr. Ron Fouchier (Erasmus Medical Center). All experimental procedures involving potential contact with MERS-CoV or SARS-CoV-2 were conducted in a biosafety level 3 (BSL3) laboratory, certified by the Korean government.
Primary human pulmonary epithelial cells were purchased from ScienCell research laboratories (Carlsbad, CA, USA) and maintained in alveolar epithelial cell medium (AEpicM) supplemented with 10% FBS.
Determination of viral titers by plaque assay
Primary human pulmonary epithelial cells, grown in 6-well plates, were infected with MERS-CoV or SARS-CoV-2 at an MOI of 0.01, and their culture supernatants were collected on days 2, 4 and 6 postinfection. Viral titers were measured by plaque assay. Briefly, serial tenfold dilutions of the collected culture supernatants were added to confluent Vero cell cultures and incubated for 4 h. Afterwards, cultures were overlaid with 1% electrophoretic agar (LPS Solution, Korea) and incubated for 4 days. Cells were then stained with 0.1% crystal violet (Sigma-Aldrich, St. Louis, MO, USA) in a 37% formaldehyde solution, and plaque-forming units (pfu) were measured.
Analysis of gene expression patterns by microarray
Primary human pulmonary epithelial cells, grown in 6-well plates, were infected with MERS-CoV or SARS-CoV-2 at an MOI of 2 for 12 hours when viral particles were produced in the infected cells. Uninfected cells were maintained under the same conditions as negative controls. Total RNA was then isolated using an RNeasy Mini Kit (QIAGEN, Hilden, Germany). The purified RNAs from infected and uninfected cells were analyzed using microarray (Affymetrix Human 2.0 ST array). This analysis was performed commercially (ebiogen, Seoul, South Korea).
RNA quality was assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies, USA), and the quantity was determined by ND-1000 spectrophotometer (NanoDrop Technologies, USA).
RNA samples were used as input in the Affymetrix procedure as recommended by the manufacturer (http://www.affymetrix.com). Briefly, total RNA from each sample was converted to double-stranded cDNA using a random hexamer incorporating a T7 promoter. Amplified RNA (cRNA) was then generated from the double-stranded cDNA template in an in vitro transcription (IVT) reaction and purified using an Affymetrix sample cleanup module. cDNA was regenerated in a random-primed reverse transcription reaction using a dNTP mix containing dUTP. The cDNA was then fragmented by UDG and APE 1 restriction endonucleases and end-labeled in a terminal transferase reaction incorporating a biotinylated dideoxynucleotide. Fragmented end-labeled cDNA was hybridized to the Affymetrix arrays for 16 h at 45 °C and 60 rpm as described in the Gene Chip Whole Transcript (WT) Sense Target Labeling Assay Manual (Affymetrix). After hybridization, the chips were stained using streptavidin phycoerythrin (SAPE), washed in a GeneChip Fluidics Station 450 (Affymetrix), and scanned by using a GeneChip Array Scanner 3000 7G (Affymetrix).
After the final wash and staining step, the Affymetrix array was scanned using an Affymetrix Model 3000 G7 scanner, and the image data were extracted using Affymetrix Command Console software 1.1. The raw.cel file generated by the above procedure contained expression intensity data and was used for the next step. Expression data were generated by Transcriptome Analysis Console 4.0.1. For normalization, the RMA (robust multi-average) algorithm implemented in the Transcriptome Analysis Console software was used.
Ethics statement
Clinical sample collection from patients was approved by the CNU hospital ethics committee. The samples were collected with the consent of the patients.
Results
Viral infectivity in primary human pulmonary epithelial cells
To evaluate if there were differences in the infectivity of MERS-CoV or SARS-CoV-2, we infected primary human pulmonary epithelial cells at an MOI of 0.01 and subsequently measured viral titers by plaque assay. Viral growth was similar. The mean viral titers of lung cells infected with MERS-CoV were 2 × 105, 9 × 105, and 9.8 × 105 pfu after 2, 4, and 6 days of infection, respectively. The mean viral titers determined for SARS-CoV-2 were 2.2 × 105, 8.5 × 105, and 9.5 × 105 at the same time points (Fig. 1).
Fig. 1.
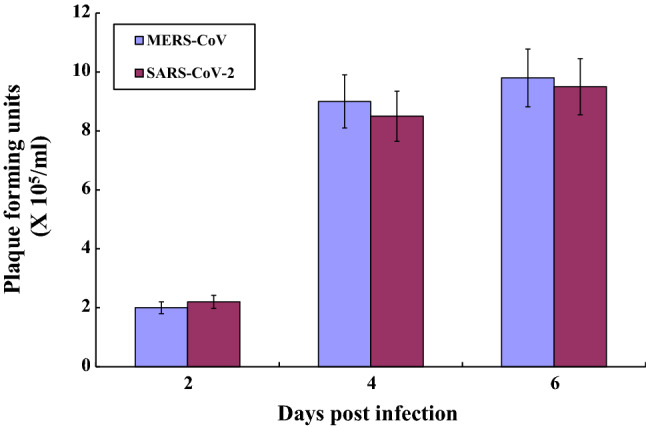
Virus growth in primary human lung epithelial cells infected with MERS-CoV and SARS-CoV-2. Primary human pulmonary alveolar cells were infected with SARS-CoV-2 or MERS-CoV at an MOI of 0.01. Viral titers were measured in Vero cells by plaque assay on days 2, 4 and 6 p.i. Data are the mean of three experiments with standard deviations
Global gene expression in primary human pulmonary epithelial cells
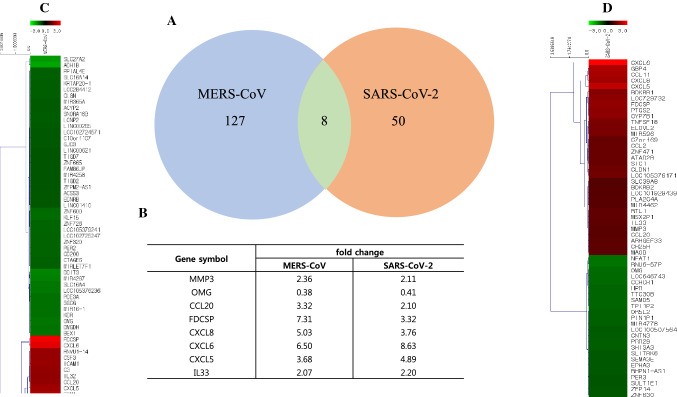
To investigate the impact of MERS-CoV and SARS-CoV-2 infection on global gene expression patterns in primary human lung cells, we infected them with each of these viruses at an MOI of 2, extracted total RNA, and performed a microarray analysis. Uninfected cells processed under the same conditions were used to define the baseline. Overall, the number of differentially expressed genes was higher in MERS-CoV-infected cells than in SARS-CoV-2-infected cells. Out of a total of 44,556 genes analyzed, 127 were differentially expressed in human lung cells infected with MERS-CoV, whereas 50 were differentially expressed in SARS-CoV-2-infected cells (Fig. 2A, C, and D). Interestingly, our convergent analysis showed that the expression of only eight genes was modulated by both coronaviruses: matrix metallopeptidase 3 (MMP3), oligodendrocyte myelin glycoprotein (OMG), CC chemokine ligand 20 (CCL20), follicular dendritic cell secreted protein (FDCSP), C-X-C ligand 5 (CXCL5), CXCL6, CXCL8 and interleukin-33 (IL-33). The differential gene expression patterns showed similar profiles in human lung cells infected by either MERS-CoV or SARS-CoV-2 (Fig. 2B). Most of the proteins encoded by these genes belong to distinct families and consequently have distinct functions, such as the proteolytic breakdown of extracellular matrix proteins (MMP3) [10], the maintenance of the structural integrity of the myelin sheath (OMG) [11], the direction of protein transport (FDCSP) [13], the chemoattraction of lymphocytes and neutrophils (CCL20, CXCL5, CXCL6 and CXCL8) [12, 14–16], or regulation of immune responses (IL-33) [17].
Fig. 2.
Heat maps and fold change of differentially expressed genes in primary human lung epithelial cells infected with MERS-CoV or SARS-CoV-2. Total RNA was collected from primary human lung epithelial cells infected with MERS-CoV or SARS-CoV-2 at 12 hours p.i. The purified RNA was used for microarray analysis using a human DNA chip. A. The number of differentially expressed genes with more than a twofold change in expression in human lung cells compared to those in uninfected human lung cells. B. Description of genes commonly modulated in human lung cells infected with MERS-CoV and SARS-CoV-2. C. Heat map of all genes with more than a twofold change in expression in human lung cells infected with MERS-CoV compared to those in uninfected human lung cells. D. Heat map of all genes with more than a twofold change in expression in human lung cells infected with SARS-CoV-2 compared to those in uninfected human lung cells
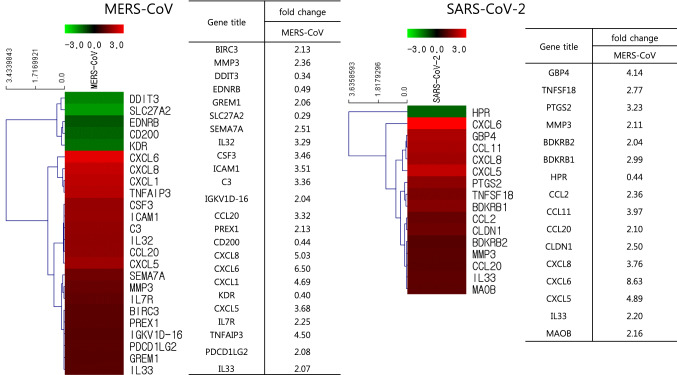
To better understand the gene expression differences in primary human pulmonary epithelial cells infected by MERS-CoV or SARS-CoV-2, we further limited our analysis and looked at genes related to cell damage, antiviral defense, immunity (senso lato), and inflammation (Table 1, Fig. 2 and Supplementary Figs. S1-S4). Out of 4443 genes, 24 and 16 were differentially expressed in cells infected with MERS-CoV and SARS-CoV-2, respectively (˃ 2-fold increase compared to uninfected cells) (Fig. 3).
Table 1.
Gene descriptions
| Gene symbol | Gene ID | Description |
|---|---|---|
| BDKRB1 | NM_000710 | Bradykinin receptor B1 |
| BDKRB2 | NM_000623 | Bradykinin receptor B2 |
| BIRC3 | NM_001165 | Baculoviral IAP repeat containing 3 |
| C3 | NM_000064 | Complement component 3 |
| CCL11 | NM_002986 | Chemokine (C-C motif) ligand 11 |
| CCL2 | NM_002982 | Chemokine (C-C motif) ligand 2 |
| CCL20 | NM_001130046 | Chemokine (C-C motif) ligand 20 |
| CD200 | NM_001004196 | CD200 molecule |
| CLDN1 | NM_021101 | Claudin 1 |
| CSF3 | NM_000759 | Colony-stimulating factor 3 |
| CXCL1 | NM_001511 | Chemokine (C-X-C motif) ligand 1 (melanoma growth stimulating activity, alpha) |
| CXCL5 | NM_002994 | Chemokine (C-X-C motif) ligand 5 |
| CXCL6 | NM_002993 | Chemokine (C-X-C motif) ligand 6 |
| CXCL8 | NM_000584 | Chemokine (C-X-C motif) ligand 8 |
| DDIT3 | NM_001195053 | DNA-damage-inducible transcript 3 |
| EDNRB | NM_000115 | Endothelin receptor type B |
| GBP4 | NM_052941 | Guanylate binding protein 4 |
| GREM1 | NM_001191322 | Gremlin 1, DAN family BMP antagonist |
| HPR | NM_020995 | Haptoglobin-related protein |
| ICAM1 | NM_000201 | Intercellular adhesion molecule 1 |
| IGKV1D-16 | OTTHUMT00000323144 | Immunoglobulin kappa variable 1D-16 |
| IL32 | NM_001012631 | Interleukin 32 |
| IL33 | NM_001199640 | Interleukin 33 |
| IL7R | NM_002185 | Interleukin 7 receptor |
| KDR | NM_002253 | Kinase insert domain receptor |
| MAOB | NM_000898 | Monoamine oxidase B |
| MMP3 | NM_002422 | Matrix metallopeptidase 3 |
| PDCD1LG2 | NM_025239 | Programmed cell death 1 ligand 2 |
| PREX1 | NM_020820 | Phosphatidylinositol-3,4,5-trisphosphate-dependent Rac exchange factor 1 |
| PTGS2 | NM_000963 | Prostaglandin-endoperoxide synthase 2 (prostaglandin G/H synthase and cyclooxygenase) |
| SEMA7A | NM_001146029 | Semaphorin 7A, GPI membrane anchor (John Milton Hagen blood group) |
| SLC27A2 | NM_001159629 | Solute carrier family 27 (fatty acid transporter), member 2 |
| TNFAIP3 | NM_001270507 | Tumor necrosis factor, alpha-induced protein 3 |
| TNFSF18 | NM_005092 | Tumor necrosis factor (ligand) superfamily, member 18 |
Fig. 3.
Heat maps and fold change of genes involved in antiviral activity, cell damage, immune response, and inflammatory response in primary human lung epithelial cells infected with MERS-CoV or SARS-CoV-2
Concerning antiviral-defense-related genes, only six were identified as differentially expressed, out of a total of 596. Of these, TNFAIP3 and guanylate-binding protein 4 (GBP4) showed the highest variation in their expression level, in cells infected with MERS-CoV (4.50-fold) and SARS-CoV-2 (4.14-fold) (Supplementary Fig. S1). Importantly, GBP4 is known to be directly involved in protective immunity against viruses [18, 19].
With respect to cell-damage-related genes, out of a total of 1115, nine and five were found to be differentially expressed in human lung cells infected with MERS-CoV and SARS-CoV-2, respectively compared to uninfected cells. Of these, tumor necrosis factor α-induced protein 3 (TNFAIP3) and prostaglandin-endoperoxide synthase 2 (PTGS2) were the ones with the highest variation in their expression level, in cells infected with MERS-CoV (4.50-fold) and SARS-CoV-2 (3.23-fold) (Supplementary Fig. S2). While TNFAIP3 is known to inhibit NF-κB activation and TNF-mediated apoptosis, PTGS2 is involved in the conversion of arachidonic acid to prostaglandin H2 [20].
Additionally, looking at immune responses as a whole (Supplementary Fig. S3), out of a total of 2044 genes involved in immunity, 20 and 10 genes were found to be differentially expressed in human lung cells infected with MERS-CoV and SARS-CoV-2, respectively compared to uninfected cells. CXCL1 and CXCL6 showed the highest variation in their expression level, in each of these contexts (4.69-fold and 8.63-fold, respectively). Both CXCL1 and CXCL6 are known to be important chemotactic factors affecting the migration of neutrophils and granulocytes [21, 22].
Out of 688 genes involved in inflammatory responses (Supplementary Fig. S4), 12 and 11 genes were differentially expressed in human lung cells infected with MERS-CoV and SARS-CoV-2, respectively, compared to the basal conditions. Because inflammation and immunity are known to overlap, CXCL6 appeared again as the gene with the highest variation in its expression level, in both MERS-CoV- and SARS-CoV-2-infected cells (8.63-fold and 6.50 fold, respectively).
Discussion
Highly pathogenic coronaviruses such as MERS-CoV, SARS-CoV, and the newly identified SARS-CoV-2 have the potential to cause severe pneumonia in humans. In this study, we used a microarray assay to analyze the global gene expression of human pulmonary epithelial cells infected with MERS-CoV or SARS-CoV-2 to investigate the impact of coronavirus infection on lung cells and, consequently, on the respiratory system as a whole.
Interestingly, we found that, out of the 44,566 human genes analyzed, more were differentially expressed in cells infected with MERS-CoV than in cells infected with SARS-CoV-2 (127 vs. 50 genes). These results may be related to the observation that MERS-CoV infections tend to be more severe than those caused by the new SARS-CoV-2. Based on the currently available data, the mortality rates for MERS-CoV and SARS-CoV-2 infections are approximately 36%, and less than 10%, respectively.
However, when we limited the analysis to inflammatory-response-related genes, the number of differentially expressed genes was similar in in vitro MERS-CoV and SARS-CoV-2 infections (12 and 11 genes, respectively). This may suggest that both infections lead to similar local inflammatory responses, which is not unexpected, considering that the two viruses are closely related and that inflammation is dependent on innate (nonspecific) immunity. On the other hand, when we looked at immune-response-related genes, the cells infected with MERS-CoV had twice as many differentially expressed genes as the cells infected with SARS-CoV-2 (20 vs. 10). These results suggest that the two infections lead to different immune responses, which are in line with previously published data. A recent study revealed that immunity-related gene expression patterns in a human lung cancer cell line (Calu-3) infected with MERS-CoV or SARS-CoV were different [23]. While the expression of genes coding for IL-1β, IL-6 and IL-8 was shown to be significantly upregulated in cells infected with MERS-CoV compared to cells infected with SARS-CoV, the reverse was observed for the expression of genes coding for TNF-α, IFN-β and IP-10 (up to 30 h postinfection) [23]. In fact, even in the context of SARS-CoV-2 infection alone, cytokine and chemokine plasma profiles have been shown to differ between patients with different levels of disease severity [24]. While IL-1β, IL-1RA, IL-8, IL-9, basic FGF, GMCSF, IFN-γ, IP-10, MIP-1β, PDGF, and VEGF concentrations were comparable between ICU- (intensive care unit) and non-ICU-admitted COVID-19 patients, the concentrations of IL-1, IL-7, IL-10, GCSF, IP-10, MCP1, MIP-1α, and TNF-α levels were higher in ICU-admitted patients than in non-ICU-admitted patients [24].
We found that CXCL6, which is a chemoattractant for neutrophilic granulocytes [15, 22], was the most strongly upregulated inflammatory gene in human pulmonary epithelial cells infected with SARS-CoV-2. CXCL6 may be involved in the development of severe pneumonia in humans infected with SARS-CoV-2 by recruiting inflammatory leukocytes into human lungs.
We used the primary human pulmonary epithelial cells to investigate changes in gene expression when SARS-CoV-2 infects lung cells. Considering that SARS-CoV-2 infects human lungs, our model might reflect some of the changes that occur in lung cells of humans infected with SARS-CoV-2. However, further in vivo study is needed to confirm our data, using human ACE (angiotensin converting enzyme)-2 transgenic mice infected with SARS-CoV-2.
In this study, we used the laboratory MERS-CoV strain EMC2012 because we did not have a recent MERS-CoV isolate. Further study may be needed to investigate gene modulation in lung cells using a more recent MERS-CoV strain.
In conclusion, the greater number of differentially expressed genes in primary pulmonary epithelial cells infected with MERS-CoV than in those infected with SARS-CoV-2 may explain the why MERS-CoV infections more frequently lead to worse outcomes than SARS-CoV-2 infections.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This manuscript was edited by an English editing company, Editage.
Compliance with ethical standards
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
Clinical sample collection from patients was approved by the CNU hospital ethics committee.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.de Wilde AH, Snijder EJ, Kikkert M, van Hemert MJ. Host factors in coronavirus replication. Curr Top Microbiol Immunol. 2018;419:1–42. doi: 10.1007/82_2017_25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020 doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nie J, Li Q, Wu J, Zhao C, Hao H, Liu H, Zhang L, Nie L, Qin H, Wang M, Lu Q, Li X, Sun Q, Liu J, Fan C, Huang W, Xu M, Wang Y. Establishment and validation of a pseudovirus neutralization assay for SARS-CoV-2. Emerg Microbes Infect. 2020;9(1):680–686. doi: 10.1080/22221751.2020.1743767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 5.Zumla A, Hui DS, Perlman S. Middle East respiratory syndrome. Lancet. 2015;386(9997):995–1007. doi: 10.1016/S0140-6736(15)60454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Q, Guan X, Wu P, Wang X, Zhou L, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phelan AL, Katz R, Gostin LO. The Novel Coronavirus Originating in Wuhan, challenges for global health governance. China: JAMA; 2020. [DOI] [PubMed] [Google Scholar]
- 8.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W. China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen N, Zhou M, Dong X, Qu J, Gong F, Ha Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Siological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emonard H, Grimaud JA. Matrix metalloproteinases. A review. Cell Mol Biol. 1990;36(2):131–153. [PubMed] [Google Scholar]
- 11.Roth MP, Malfroy L, Offer C, Sevin J, Enault G, Borot N, Pontarotti P, Coppin H. The human myelin oligodendrocyte glycoprotein (MOG) gene: complete nucleotide sequence and structural characterization. Genomics. 1995;28(2):241–250. doi: 10.1006/geno.1995.1137. [DOI] [PubMed] [Google Scholar]
- 12.Hieshima K, Imai T, Opdenakker G, Van Damme J, Kusuda J, Tei H, Sakaki Y, Takatsuki K, Miura R, Yoshie O, Nomiyama H. Molecular cloning of a novel human CC chemokine liver and activation-regulated chemokine (LARC) expressed in liver. Chemotactic activity for lymphocytes and gene localization on chromosome. J Biol Chem. 1997;272(9):5846–5853. doi: 10.1074/jbc.272.9.5846. [DOI] [PubMed] [Google Scholar]
- 13.Marshall AJ, Du Q, Draves KE, Shikishima Y, HayGlass KT, Clark EA. FDC-SP, a novel secreted protein expressed by follicular dendritic cells. J Immunol. 2002;169(5):2381–2389. doi: 10.4049/jimmunol.169.5.2381. [DOI] [PubMed] [Google Scholar]
- 14.Hedges JC, Singer CA, Gerthoffer WT. Mitogen-activated protein kinases regulate cytokine gene expression in human airway myocytes. Am J Respir Cell Mol Biol. 2000;23(1):86–94. doi: 10.1165/ajrcmb.23.1.4014. [DOI] [PubMed] [Google Scholar]
- 15.Proost P, Wuyts A, Conings R, Lenaerts J, Billiau A, Opdenakker G, Van Damme J. Human and bovine granulocyte chemotactic protein-2: complete amino acid sequence and functional characterization as chemokines. Biochemistry. 1993;32(38):10170–10177. doi: 10.1021/bi00089a037. [DOI] [PubMed] [Google Scholar]
- 16.Chang MS, McNinch J, Basu R, Imonet S. Cloning and characterization of the human neutrophil-activating peptide (ENA-78) gene. J Biol Chem. 1994;269(41):25277–25282. [PubMed] [Google Scholar]
- 17.Yagami A, Orihara K, Morita H, Futamura K, Hashimoto N, Matsumoto K, et al. IL-33 mediates inflammatory responses in human lung tissue cells. J Immunol. 2010;185(10):5743–57450. doi: 10.4049/jimmunol.0903818. [DOI] [PubMed] [Google Scholar]
- 18.Staal J, Driege Y, Haegman M, Borghi A, Hulpiau P, Lievens L, et al. Ancient origin of the CARD-coiled coil/Bcl10/MALT1-like paracaspase signaling complex indicates unknown critical functions. Front Immunol. 2018;9:1136. doi: 10.3389/fimmu.2018.01136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tripal P, Bauer M, Naschberger E, Mortinger T, Hohenadl C, Cornali E, Thurau M, Sturzl M. Unique features of different members of the human guanylate-binding protein family. J Interferon Cytokine Res. 2007;27(1):44–52. doi: 10.1089/jir.2007.0086. [DOI] [PubMed] [Google Scholar]
- 20.Hla T, Neilson K. Human cyclooxygenase-2 cDNA. Proc Natl Acad Sci USA. 1992;89(16):7384–7388. doi: 10.1073/pnas.89.16.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Becker S, Quay J, Koren HS, Haskill JS. Constitutive and stimulated MCP-1, GRO alpha, beta, and gamma expression in human airway epithelium and bronchoalveolar macrophages. Am J Physiol. 1994;266(3 Pt 1):L278–L286. doi: 10.1152/ajplung.1994.266.3.L278. [DOI] [PubMed] [Google Scholar]
- 22.Wuyts A, Van Osselaer N, Haelens A, Samson I, Herdewijn P, Ben-Baruch A, Oppenheim J, Proost P, Van Damme J. Characterization of synthetic human granulocyte chemotactic protein 2: usage of chemokine receptors CXCR1 and CXCR2 and in vivo inflammatory properties. Biochemistry. 1997;36(9):2716–2723. doi: 10.1021/bi961999z. [DOI] [PubMed] [Google Scholar]
- 23.Lau SKP, Lau CCY, Chan KH, Li CPY, Chen H, Jin DY, Chan JFW, Woo PCY, Yuen KY. Delayed induction of proinflammatory cytokines and suppression of innate antiviral response by the novel Middle East respiratory syndrome coronavirus: implications for pathogenesis and treatment. J Gen Virol. 2013;94(Pt 12):2679–2690. doi: 10.1099/vir.0.055533-0. [DOI] [PubMed] [Google Scholar]
- 24.Huang C, Wang Y, Li X, Ren L, Zhao J, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.