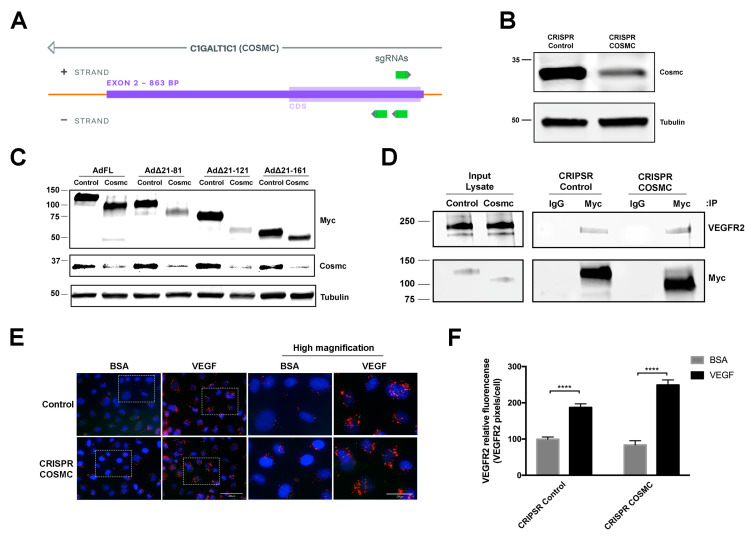
Figure 4.
O-glycosylation of EMCN is not required for modulating VEGFR2 signaling. (A) Design of the CRISPR-Cas9 for targeted COSMC knockout in HREC. (B) Whole cell lysates from CRIPSR control and COSMC knockout (CRIPSR Cosmc) were extracted and processed for western blot, which showed approximately 70% reduction in COSMC protein levels; n = 3 (C) Whole cell lysate samples were collected 72 h post-transduction. Molecular weight of the Myc-tagged FL EMCN and EMCN mutants, with and without mucin-type O-glycosylation, were examined by western blot using anti-Myc tag antibody. Protein levels of COSMC revealed a significant reduction of COSMC at the protein level, and α-tubulin was used as loading control, confirming the reduction of COSMC. Examination by western blot of FL EMCN, ∆21-81 EMCN, ∆21-121 EMCN, and ∆21-161 EMCN revealed molecular weight reductions of ~30 kDa, ~25 kDa, ~20 kDa, and ~10 kDa, respectively, consistent with reduced O-glycosylation; n = 3 (D) The levels of VEGFR2 co-IP’ed with EMCN in CRIPSR control HRECs or COSMC knockout HRECs (CRIPSR Cosmc) and examined by western blot indicate that the lack of O-glycosylation did not impact the interaction of EMCN and VEGFR2; n = 3 (E,F) To examine VEGFR2 internalization, CRIPSR control HRECs or COSMC knockout HRECs were stimulated with VEGF or BSA and VEGF2 tracked with an antibody that recognizes the VEGFR2 extracellular domain (ECD). VEGF stimulated VEGFR2 internalization in both cells, indicating that O-glycosylation of EMCN is not necessary for VEGFR2 internalization. Boxes areas in the left-hand panel are shown magnified in the right-hand panels. Lower magnification, scale bar: 40 μm, higher magnification, scale bar: 20 μm. (F) The levels of Internalized VEGFR2 were quantified as VEGFR2 pixels divided by the number of cells per images. Data = mean ± SEM, **** p < 0.0001 by 2-tail unpaired t-test, n = 6.