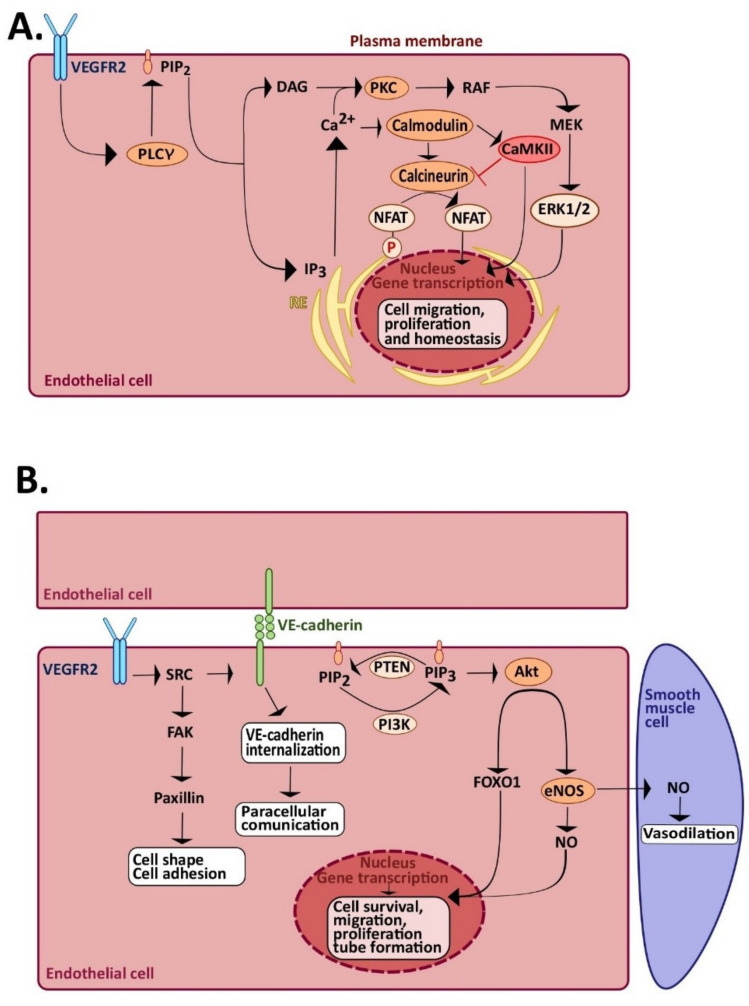
Figure 2.
Role of endothelial Ca2+ signaling angiogenesis and vasculogenesis. (A) VEGF binds to VEGFR2 and induces receptor dimerization and transphosphorylation. Activation of VEGFR2, in turn, leads to InsP3-dependent Ca2+ release, followed by SOCE activation (not shown). The ensuing increase in [Ca2+]i leads to the activation of Ca2+-dependent decoders, such as calmodulin, which in turn activates calcineurin and Ca2+-Calmodulin-dependent kinase II (CaMKII). Calcineurin dephosphorylates NFAT, thereby promoting its translocation into the nucleus, where it activates genes responsible for cell proliferation and migration. CaMKII, in turn, inhibits calcineurin activity, but stimulates angiogenesis by phosphorylating multiple targets (i.e., Akt and Src, not shown). In addition, the increase in [Ca2+]i promotes cytosolic protein kinase C (PKC) relocation toward the plasma membrane. Herein, PKC is activated by DAG to stimulate the extracellular signal-regulated kinases 1/2 (ERK1/2) phosphorylation cascade. (B) Schematic representation of the Src and PI3K-Akt signaling pathways activated by VEGF. Once activated, VEGFR2 activates Src and subsequent downstream pathways involved in cell shape, adhesion, permeability, and survival. Moreover, VEGFR2 indirectly activates PI3K, either by Src or by VE-cadherin. PI3K generates phosphatidylinositol-3,4,5-trisphosphate (PIP3), which activates Akt, followed by eNOS activation and NO release. Finally, Akt activates FOXO1, which translocates into the nucleus, where, together with NO, induces transcription of genes involved in cell survival, migration, proliferation, and tube formation.