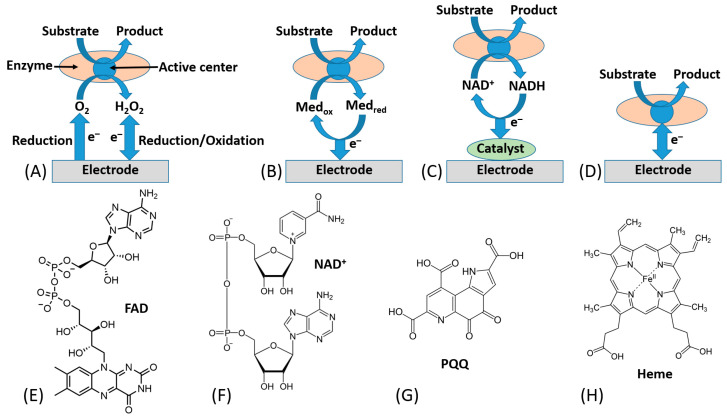
Figure 2.
Different ways of electronic communication between redox enzymes and conductive electrodes: (A) Electrical communication through electrochemical transformations of enzyme substrate or product (exemplified with reduction of O2 and reduction/oxidation of H2O2 typical for oxidases). (B) Electrical communication using electron transfer mediators (relays) cyclic between oxidized (Medox) and reduced (Medred) states (exemplified with an enzyme oxidizing a substrate and reducing a mediator, which is electrochemically re-oxidized and recycled back to Medox). (C) Electrical communication using NAD+/NADH cofactor re-oxidized and recycled electrocatalytically (exemplified with an enzyme oxidizing a substrate and reducing NAD+ yielding NADH). (D) Electrical communication via direct electron transfer (DET) from an enzyme active center to an electrode (exemplified with an enzyme oxidizing a substrate and generating anodic current at an electrode). (E–H) Structures of the most typical enzyme redox cofactors: flavin adenine dinucleotide (FAD), nicotinamide adenine dinucleotide (NAD+), pyrroloquinoline quinone (PQQ) and heme.