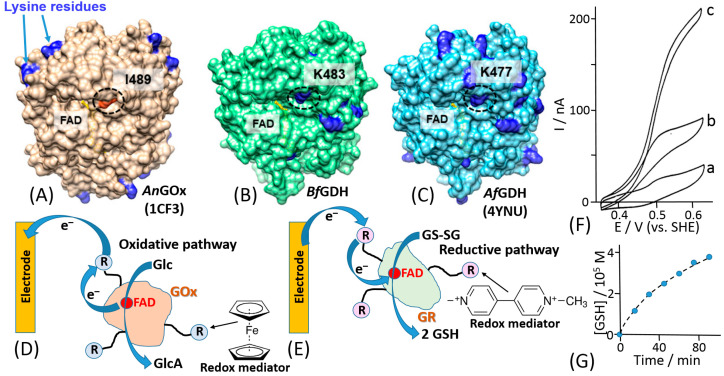
Figure 4.
(A–C) Comparison of the positions of lysine residues in Aspergillus niger derived glucose oxidase (AnGOx) (PDB ID: 1CF3), Botryotinia fuckeliana derived glucose dehydrogenase (BfGDH) (model), and A.flavus derived GDH (AfGDH) (PDB ID: 4YNU). Lysine residues are shown in dark blue. In BfGDH and AfGDH, lysine residues (K483, K477, circled) are located at the entrance of what appears to be a pathway to the active center. In AnGOx, an isoleucine residue (I489, circled) is located at this position. (D) The electron transfer from soluble GOx to a Au electrode mediated by ferrocene redox relay (R) species covalently tethered to the enzyme with long flexible chains. Note that ferrocene has a positive redox potential needed to mediate the oxidative biocatalytic process. The biocatalytic reaction results in glucose (Glc) oxidation and gluconic acid (GlcA) formation. (E) The electron transfer from a Au electrode to soluble glutathione reductase (GR) mediated by viologen redox relay species covalently tethered to the enzyme with long flexible chains. Note that viologen has a very negative redox potential needed to mediate the reductive biocatalytic process. The biocatalytic reaction results in transformation of the oxidized glutathione (GS-SG) to the reduced glutathione (G-SH). (F) Cyclic voltammograms obtained with a bare (unmodified) Au electrode (a disk of 1.5 mm diameter) measured in the presence of a ferrocene-functionalized GOx (12 ferrocene electron relays per a GOx molecule, shown schematically in (D); 10 mg/mL): (a) in the absence of glucose; (b) and (c) in the presence of 0.8 mM and 5 mM glucose, respectively. A phosphate buffer solution (0.085 M, pH 7.0) was used as a background electrolyte applied under N2 atmosphere. Scan rate was 2 mV/s. (G) Formation of reduced glutathione bioelectrocatalyzed by GR functionalized with viologen mediator units tethered to the enzyme with long flexible chains (see experimental details in [48]. (Part A is adopted from ref. [46] with permission; part F is adopted from [20] with permission.)