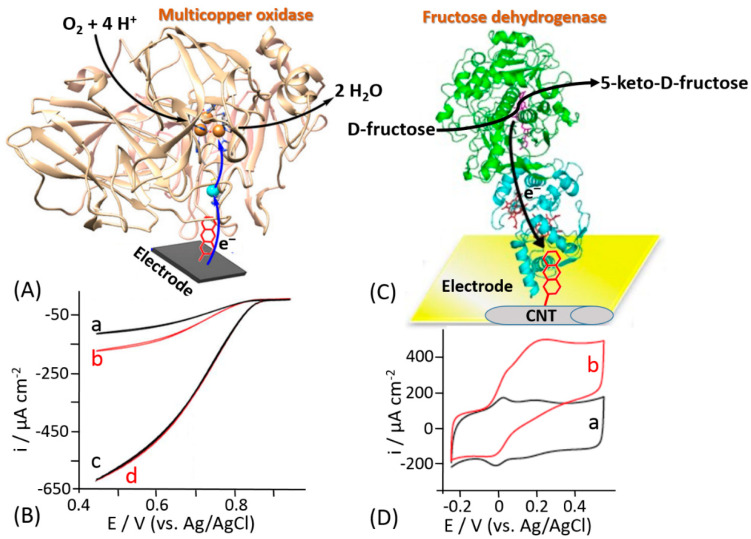
Figure 15.
(A) Laccase (multicopper oxidase) site-specific immobilization due to the enzyme binding to the surface-located aromatic species. (B) The electrocatalytic activity of a film of Pycnoporus cinnabarinus laccase lcc3-1 (PcL) on an electrode without aromatic species and random orientation of the adsorbed enzyme (a–b) and on an electrode functionalized with the aromatic species providing orientation of the enzyme favorable for the DET (c–d). Cyclic voltammograms (a) and (c) correspond to the catalytic waves immediately after spotting on laccase solution. Cyclic voltammograms (b) and (d) show the catalytic waves after additional treatment of the modified electrode with a new portion of the enzyme. Potential scan rate was 5 mV s−1. (C) Fructose dehydrogenase site-specific immobilization due to the enzyme binding to the surface-located aromatic species. (D) Cyclic voltammograms measured with fructose dehydrogenase-electrode modified according the scheme shown in (C); in the absence (a) and presence of 10 mM D-fructose (b). The background electrolyte was 50 mM acetic buffer, pH 4.5; potential scan rate was 10 mV s−1. (Part A was adopted from [3] with permission; part B was adopted from [170] with permission; part D was adopted from [178] with permission.)