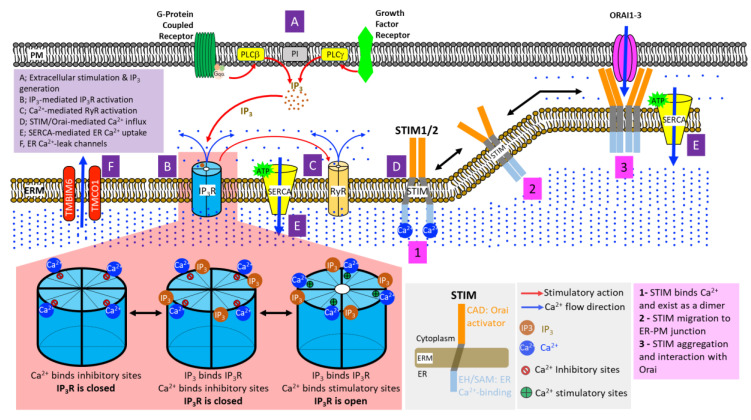
Figure 2.
Mechanisms of ER Ca2+ handling. (A) Stimulations of G-protein Coupled Receptors (GPCRs) and Receptor Tyrosine Kinases (RTKs) signal to phospholipase C-beta (PLC-beta) and PLC-gamma at the plasma membrane, respectively. This leads to PLC-mediated hydrolytic cleavage of phosphatidylinositol 4,5-bisphosphate, producing the Ca2+-mobilizing inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG) (not shown in the figure) at the cell membrane. (B) Four molecules of IP3 bind to the tetrameric IP3 receptors (IP3Rs) on the ER membrane, exposing their stimulatory Ca2+ binding sites while simultaneously obstructing inhibitory Ca2+ binding sites. Upon the co-binding of Ca2+ and IP3, IP3R channel pore opens, initiating ER Ca2+ release. (C) Elevated cytosolic Ca2+ further induces the opening of ryanodine receptors (RyRs) on the ER membrane, causing rapid and massive influx of ER Ca2+ into the cytosol. (D) Dwindling luminal ER Ca2+ results in the oligomerization of EF-SAM domain of stromal interaction molecules (STIMs), which, in turn, induces the multimerization of cytoplasmic STIM domains followed by translocation and assembly of STIM clusters at the ER-plasma membrane (ER-PM) junctions. In direct physical association with Orai channels on the plasma membrane, STIM clusters induce the opening of Orai channel pore, allowing extracellular Ca2+ entry into the cytosol. (E) Powered by ATP hydrolysis, the Sarco/Endoplasmic Reticulum Ca2+-ATPases (SERCAs) shuttle the influx of extracellular Ca2+ into the ER or SR, restoring cellular Ca2+ homeostasis. (F) ER Ca2+-leak channels, such as TMBIM6 and TMCO1, prevent ER Ca2+ over-filling.