Figure 2.
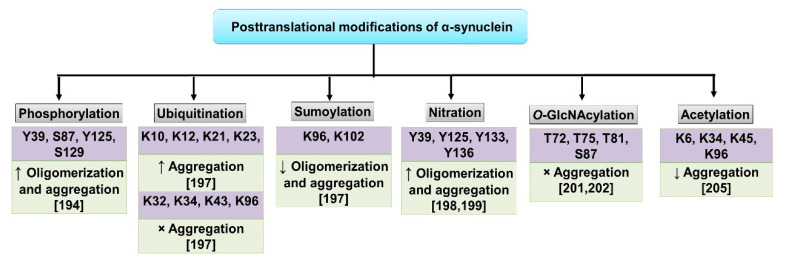
Posttranslational modifications identified that regulate α-synuclein function: Several posttranscriptional modifications (PTMs) have been reported that regulate proteostasis of α-synuclein such as phosphorylation, ubiquitination, sumoylation, nitration, acetylation and O-GlcNAcylation. α-synuclein is reported to be phosphorylated at serine (S129 and S87) [195] and tyrosine (Y125, Y133 and Y135) residues [196]. α-synuclein associated with Lewy bodies (LBs) is majorly phosphorylated at S129 [197]. Phosphorylation is catalyzed by several kinases such as Casein kinases (CKs), Polo-like kinases (PLKs) and G-protein coupled receptor kinases (GRKs) [25]. α-synuclein is ubiquitinated by the E3 ubiquitin-protein ligases like C-terminus of Hsp70 Interacting Protein (CHIP), Seven In Absentia Homologue (SIAH) and Nedd4 at various residues that either increase or stop the aggregation of the protein [198]. SUMOylation (SUMO: small ubiquitin-like modifier) of α-synuclein at residues K96 and K102 by PIAS2 that reduces oligomerization and aggregation was reported [199]. Nitration of tyrosine residues Y39, Y125, Y133 and Y136 results in increased oligomerization and aggregation of the protein [200,201], Enhanced fibrillization was also reported upon nitration of Y39 [202]. O-GlcNAcylation on the other hand inhibits toxicity caused by extracellular α-synuclein aggregates [203,204]. α-synuclein under physiological conditions is reported to undergo N-terminal acetylation. This modification most likely keeps the protein in its native form and prevents/reduces aggregation. K6, K34, K45 and K96 are reported to undergo acetylation [205]. Several of these PTMs may influence toxicity and aggregation of the protein. α-synuclein may also have several of the abovementioned PTMs at a given time in vivo. ↑—increase, ↓—decrease and ×—stops.