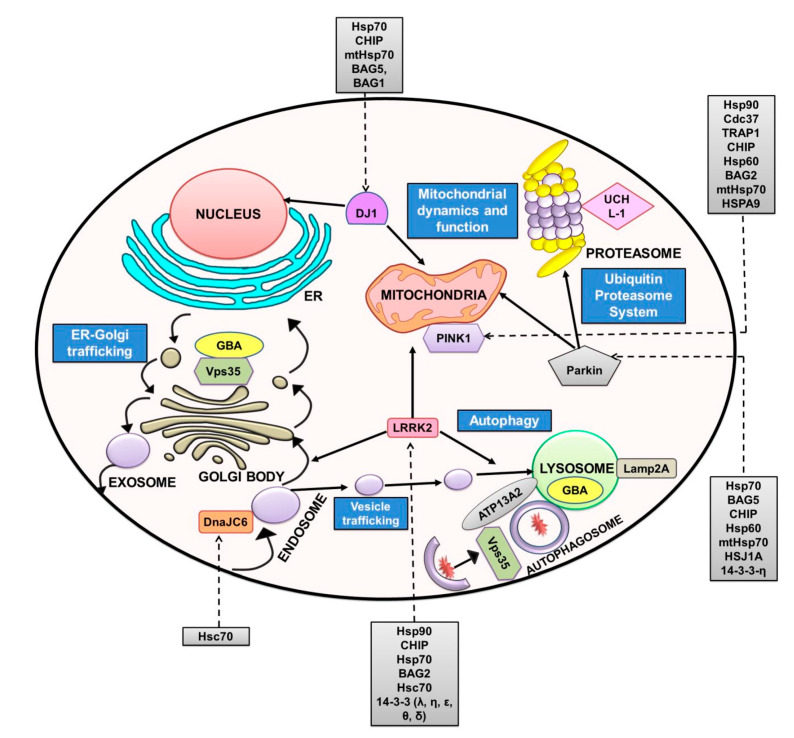
Figure 3.
PD-related proteins and chaperones involved in proteostasis: Other well-characterized PD-related proteins, their cellular localization and functional pathways involved are depicted in this figure. These include leucine-rich repeat kinase 2 (LRRK2; PARK8), PTEN-induced putative kinase 1 (PINK1; PARK6), Parkin (PARK2), DJ-1 (PARK7), glucocerebrosidase (GBA), Vps35, UCH-L1 and ATP13A2. A role for LRRK2 in vesicular trafficking, mitochondrial dynamics and lysosome-autophagy pathways has been reported [206]. Mutations in LRRK2 associated with PD and perturb functions related to above cellular pathways are identified. PINK1/Parkin-mediated mitophagy has been extensively characterized, and mutations in these proteins are associated with PD. Parkin also induces protein degradation via UPS [207]. DJ-1 regulates mitochondrial function during oxidative stress and is also reported to localize to the nucleus [155,156]. Vps35 is a component of the retromer complex and has a role in endosomal trafficking and autophagy-mediated degradation [208]. GBA is an active lysosomal enzyme involved in the degradation of complex molecules and mutations in GBA are likely to accumulate toxic α-synuclein aggregates [208]. Another important protein is associated with lysosomal dysfunction, and therefore, cell viability is ATP13A2. Mutations in this protein are identified in PD patients [4]. Ubiquitin C-terminal Hydrolase L1 (UCH-L1) is involved in the UPS pathway of proteostasis, and mutations in this protein leading to PD pathogenesis have been identified [66]. Several DNAJ proteins that belong to the Hsp40 family are studied for their role in PD. Mutations in one of these chaperones, DNAJC6, resulted in the onset of juvenile PD [177]. Most of these proteins interact with chaperones to maintain their cellular homeostasis and.