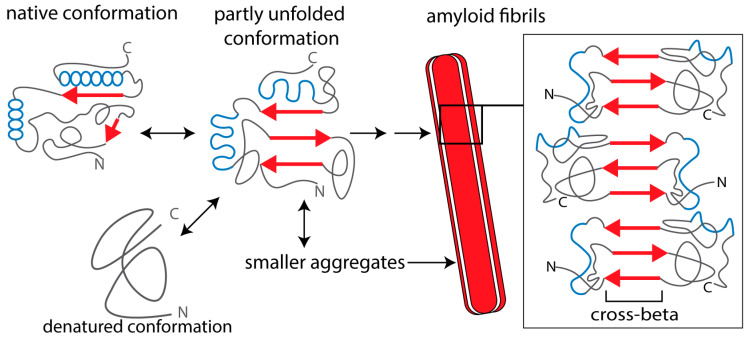
Figure 1.
Schematic representation of amyloid protein aggregation. The β-strands in the amyloid-forming protein are indicated as red arrows and α-helices as blue spheres. In the native conformation, β-strands of the monomeric protein (if present) are not aligned and “shielded”, which prevents intermolecular aggregation. A partially unfolded or misfolded molecule can form different kinds of intermolecular aggregates. Amyloid oligomers are relatively small, compact structures that may be composed of antiparallel β-strands or contain α-helical conformations. Protofibrils and mature amyloid fibrils are formed via β-strand stacking, forming extended networks of β-sheets with a characteristic cross-beta structure. Mature fibrils consist of a few identical fibrillar “subunits”. Smaller aggregates (oligomers and protofibrils) are mostly cytotoxic, whereas extracellular, fibrillar amyloid deposits can also impair tissue and organ function by impairing blood supply to the cells (see text for references).