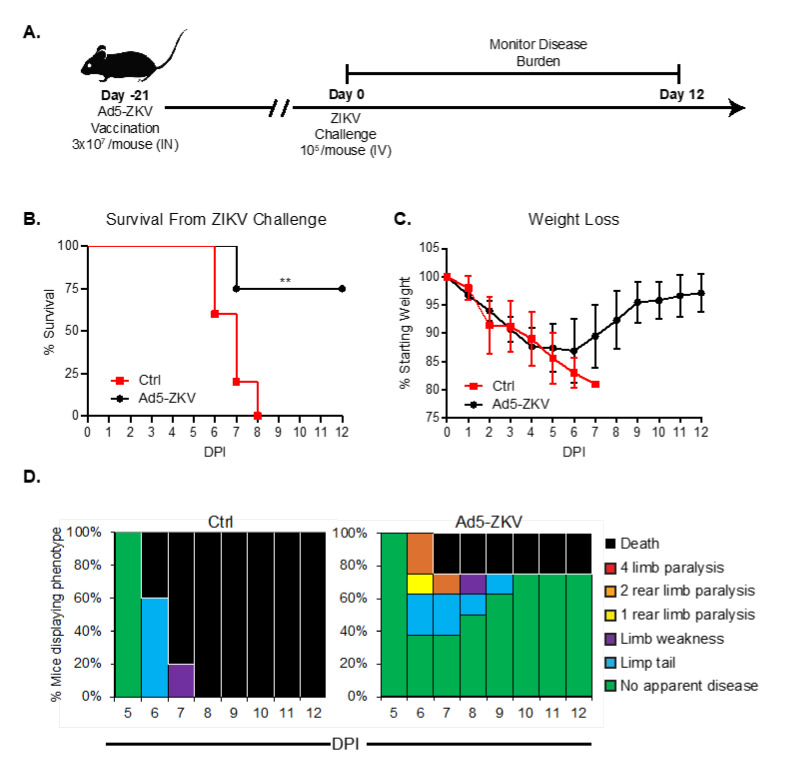
Figure 4.
Protective Capacity of hAd5-ZKV vaccine vector encoding CD8+ T cell and B cell epitopes. (A) Graphical timeline for hAd5-ZKV vaccination. 3 × 107 viral particles of hAd5-ZKV were administered intranasally to Ifnar1-/- mice. Twenty-one days later, both the vaccinated animals and a PBS-vaccinated control group were sub-lethally challenged with 1 × 104 FFU i.v. of ZIKV PRVABC59, and weight loss was monitored. (B) ZIKV disease-associated clinical scores were monitored over the twelve-day period post challenge with n = 8 for vaccination and n = 5 for control. Statistical significance for survival (** p = 0.002) was determined using a log-rank (Mantel–Cox) test. (C) ZIKV-induced weight loss was monitored over the twelve-day period post challenge. Data are the cumulative results of two independent experiments. (D) Clinical disease score over the twelve-day period post challenge with ZIKV. Phenotypes evaluated included limp tail, limb weakness, limb paralysis, and death. Data are the cumulative results of two independent experiments.