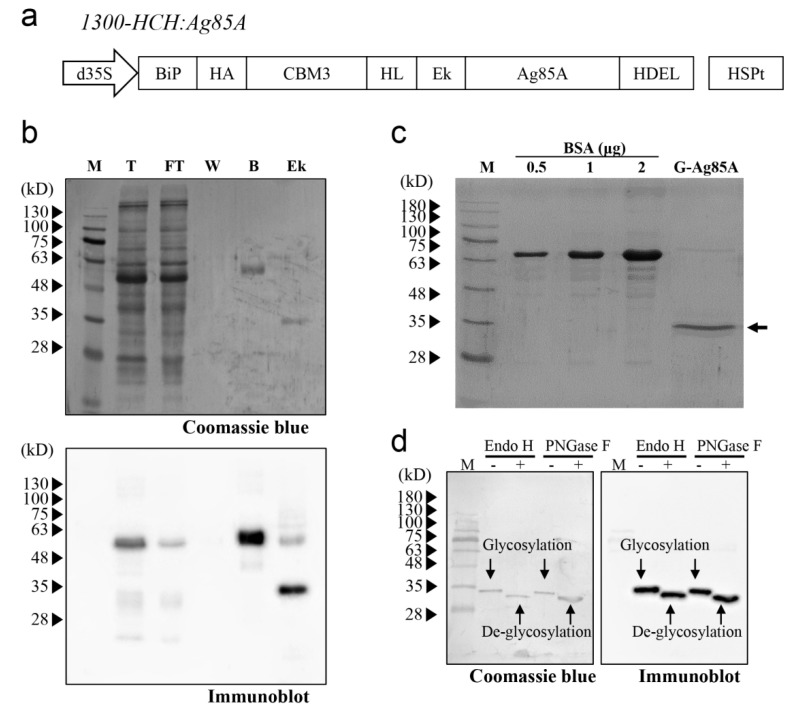
Figure 1.
Expression and purification of Ag85A in N. benthamiana. (a) Schematic representation of the recombinant Ag85A fusion construct. The fusion construct contains a BiP signal peptide, HA tag, CBM3, helical linker (HL), enterokinase cleavage site (Ek), Ag85A, and ER retention signal HDEL. This fusion construct was under the control of the cauliflower mosaic virus containing two enhancers, a 35S promoter and a heat shock protein (HSP) terminator. (b) Binding of the recombinant Ag85A fusion protein to MCC beads and enterokinase-mediated cleavage. The protein samples for each fraction were analyzed by western blotting using an anti-Ag85A antibody. M, protein standard marker; T, total; FT, flow-through; W, washing; B, MCC beads; Ek, enterokinase-treated supernatant and beads. (c) Western blot analysis of purified G-Ag85A. The purified G-Ag85A protein was analyzed by SDS-PAGE. BSA was loaded together to check the amount of G-Ag85A protein. (d) Deglycosylation of G-Ag85A. Purified G-Ag85A was treated with Endo-H or PNGase F and subjected to SDS-PAGE together with untreated G-Ag85A and then to western blot analysis using an anti-Ag85A antibody.