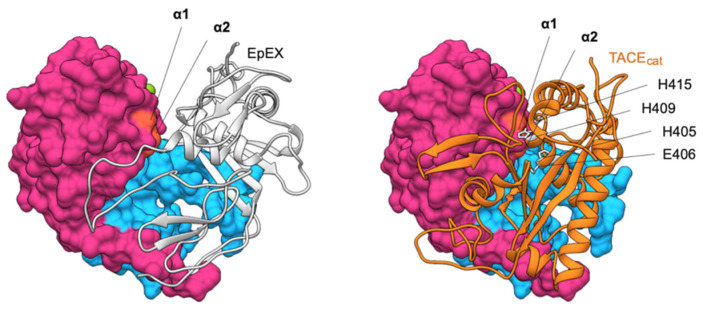
Figure 8.
Comparison of EpEX cis-dimer structure (left) and model of EpEX–TACEcat complex (right). One subunit of the dimer (gray ribbon) covers the cleavage site within the other subunit (molecular surface, cleavage site in orange). This cleavage site is in EpEX monomer easily accessible as shown by the complex of one subunit with a catalytic domain of TACE (TACEcat; orange ribbon). The model was generated using HADDOCK [67] with α1 and α2 cleavage sites on EpEX (orange surface) or TACE active and zinc binding site (H405, E406, H409, and H415; gray side chains) used as interaction restraints.