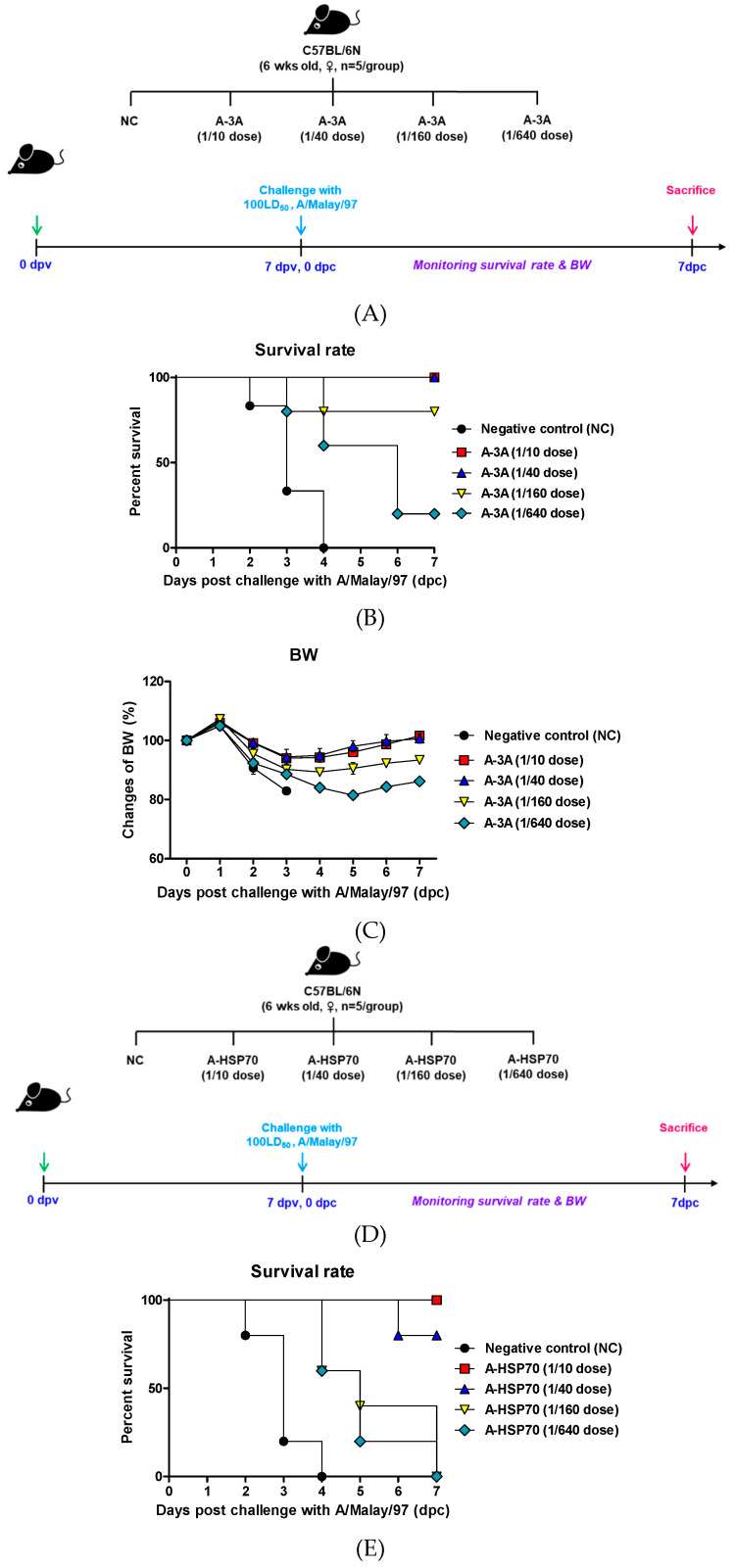
Figure 2.
A-3A- and A-HSP70-mediated vaccine efficacy and protective effects in mice. (A–F) represent: (A–C) A-3A; (D–F) A-HSP70. C57BL/6 mice were administered the test vaccine at 1/10, 1/40, 1/160, 1/640 doses of A-3A or A-HSP70 antigen for cattle or pig use, ISA 206 (oil-based emulsion, 50%, w/w), 10% Al(OH)3, and 15 µg Quil-A. A negative control (NC) group was injected with the same volume of PBS. The test vaccines were injected intramuscularly into mice that were later challenged with FMDV (100 LD50 A/Malay/97) at 7 dpv. The survival rates and body weights were monitored for 7 dpc. Experimental strategy (A,D); survival rates post-challenge with A/Malay/97 (B,E); and changes in body weight post-challenge with A/Malay/97 (C,F). The data represent the mean ± SEM of triplicate measurements (n = 5/group). Statistical analyses were performed using two-way ANOVAs with a Bonferroni correction and a one-way ANOVA followed by a Tukey’s post-hoc test. ns, not significant. Statistical analyses are summarized in Table S2.