Figure 3.
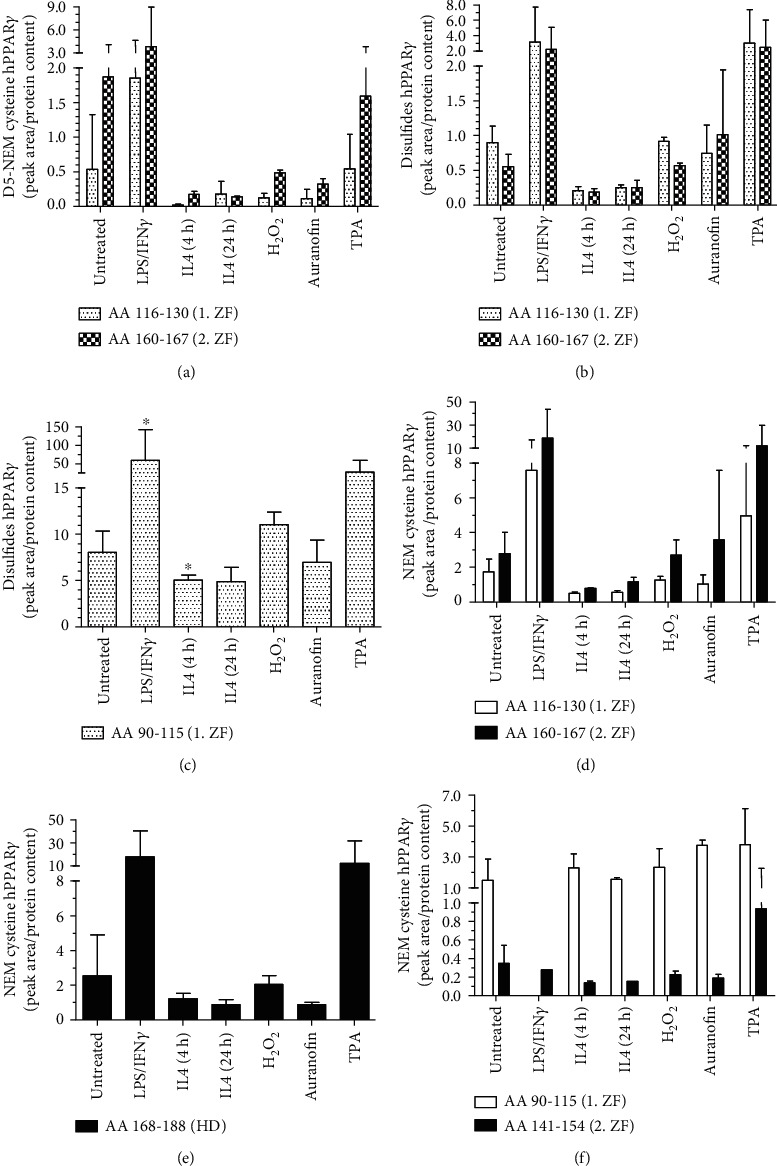
Determination of the redox status of hPPARγ cysteines via mass spectrometric analysis. J774A.1 cells were transduced using lentiviral particles with integrated N- and C-terminal HA-labeled hPPARγ. To induce redox stress, MΦ were treated by 1 μg/ml LPS combined with 10 U/ml IFNγ for 4 h, 10 ng/ml IL4 for 4 h and 24 h, H2O2 (100 μM, 15 min), auranofin (3 μM, 40 min), or TPA (1 μM, 30 min). Untreated cells were used as controls. The redox states of reduced cysteines were fixed by NEM before cell harvest on ice. HA-tagged hPPARγ was HA-immunoprecipitated, existing oxidized cysteines denatured and labeled by D5-NEM. Peptides were analysed by LC/MS after LysC digestion. Quantification of cellular oxidized (D5 NEM, disulfides) and reduced (NEM) cysteine each with respect to the corresponding protein concentrations of the sample (Supplementary Figure 4) are mapped (a–f). Peptides of different hPPARγ amino acid sequences (AA) are depicted in Table 1. Cysteines affiliations to the first and second zinc finger (ZF) and hinge domain (HD) are indicated. All experiments were performed at least three times. Mean values ± SD are provided. (∗p ≤ 0.05).
