Abstract
Background:
The unmet need for predictive biomarkers emerged from the unpredictable pattern of response to androgen signaling inhibition in metastatic castration-resistant prostate cancer (mCRPC). Here, we report on the testing of a previously identified candidate androgen signaling signature associated with response to androgen signaling inhibition.
Patients and methods:
We report on the outcome of the first module of a phase II trial on Abiraterone acetate (AA) followed by combination with dasatinib or sunitinib. Bone marrow biopsies (BMB) with matched bone marrow aspirate and blood samples were collected at baseline and upon progression. Endpoints included assessment of a prespecified molecular signature consisting of nuclear androgen receptor (AR) overexpression, cytochrome P450, family 17, subfamily A, polypeptide 1 (CYP17) expression, and AR C-/N-terminal expression ratio of ≥0.8 by immunohistochemistry (IHC) in patients with benefit versus primary resistance to AA (i.e. progression within 4 months). Tumor markers also included v-ets avian erythroblastosis virus E26 oncogene homolog (ERG), splice variant ARV7 by IHC and steroids by Liquid chromatography-tandem mass spectrometry.
Results:
Out of 170 patients accrued from 03/2011 to 02/2015, 44 (26%) were primary resistant to AA. 48 patients had tumor infiltrated BMB at baseline. Pretreatment androgen signaling signature was linked to benefit from AA (p <0.001). Presence of ERG was associated with benefit (p=0.05), while nuclear ARV7 presence and 20 or more bone lesions at baseline with primary resistance (p=0.04 and p=0.0006 respectively).
Conclusion:
Testing of a prespecified androgen signaling signature was highly supportive of its predictive value in maximal androgen deprivation strategies in mCRPC. Further validation is under way.
Trial registration:
Keywords: Castration-resistant prostate cancer, Bone metastasis, Androgen receptor, Predictive biomarkers, Abiraterone acetate
1. Introduction
In the past decade, the introduction of novel androgen signalling targeting agents in prostate cancer therapeutics has led to impressive drug development. To date, 12 phase III studies have reported positive outcomes across the disease spectrum [1–12]. Even though these agents exhibit efficacy both in castration naïve and resistant disease, there is a subset of tumors exhibiting primary resistance. The castration resistant state is more enriched for these, with roughly one of four patients exhibiting primary resistance to treatment with abiraterone acetate (AA) or enzalutamide [13, 14].
Notwithstanding the impressive drug development, therapy development has been limited by a prevailing ‘one fits all’ culture. Hence, even though we are aware that response to novel androgen signaling inhibitors is not universal, we invariably expose all patients to novel androgen signaling inhibitors, thus incurring detrimental delays and unwarranted safety risks. The unmet need for markers predictive of outcome has been our primary focus with a series of studies in patients with metastatic castration resistant prostate cancer (mCRPC). We have demonstrated the feasibility of serial bone marrow sampling in the clinical setting and identified candidate molecular markers predictive of response to therapy. This included the first in human report of association of nuclear androgen receptor splice variant 7 (ARV7) presence with primary resistance [14]. Moreover, androgen receptor (AR) overexpression, CYP17 expression and an AR-C /-N terminal domain expression ratio of approximately 1 were associated with response to both AA and enzalutamide [13–15]. This prespecified molecular signature was prospectively tested in a bi-modular phase II trial in patients with bone mCRPC receiving maximal androgen depletion with AA (1st module) followed by randomization to combination with dasatinib or sunitinib (2nd module). We present results of the 1st module, testing the proposed candidate androgen signaling signature.
2. Methods
2.1. Patients
A prospective, open-label, phase II, randomized, single-center study was conducted at MD Anderson Cancer Center (MDACC) following institutional review board approval (NCT01254864). Patients had mCRPC progression defined as prostate specific antigen (PSA) progression per Prostate Cancer Working Group (PCWG2) criteria or radiographic progression with castrate testosterone levels ≤ 50 ng/dL [16]. Previous treatment with docetaxel was allowed. All patients provided written informed consent. A comprehensive list of inclusion and exclusion criteria is available in the supplementary material.
2.2. Study Design
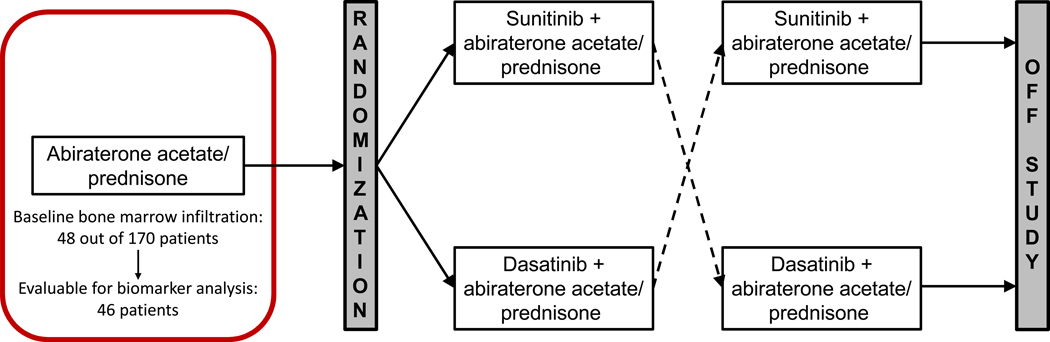
This is a phase II study of AA in mCRPC patients randomized to combination with dasatinib or sunitinib upon progression. Patients received treatment with 1g AA orally once daily in combination with 5mg prednisone orally twice daily (1st module of the trial). Treatment continued until “composite” progression according to PCWG2 criteria. At the time of progression, patients were evaluated and randomized to receive Sunitinib or Dasatinib while continuing AA (2nd module of the trial). Patients continued treatment until progression per PCWG2 criteria or limiting toxicity and subsequently crossed over to the alternate targeted agent. Study design is depicted in Figure 1.
Figure 1: Trial schema.
We are reporting the 1st module of the trial. Assessments included bone marrow biopsy (BMB) with matched blood and bone marrow aspirate (BMA) collection at baseline and upon progression on AA.
A key study objective, evaluable from the first module of the trial and the subject of this report, was testing a prespecified candidate androgen signalling signature in patients with benefit versus primary resistance to AA, defined as “composite” progression within 4 months.
The proposed molecular signature tested consists of nuclear AR-N terminal overexpression (>75% tumor involvement) plus cytoplasmic CYP17 expression (defined as >10% tumor involvement) plus a ratio of AR-C terminal / AR-N terminal domain expression ≥ 0.8 as defined during exploratory phase trials [13–15]. We also searched for potential associations of outcome with the expression of nuclear ARV7, glucocorticoid receptor (GR), ERG, Ki67, phospho-Src, vascular endothelial growth factor (VEGF), as well as with the steroid metabolome as predefined in the study.
2.3. Specimen handling and storage and Assay methodologies
Specimen collection, handling and storage as well immunohistochemistry (IHC) and liquid chromatography-mass spectrometry (LCMS) were performed as previously described [13–15, 17]. Detailed methodology description in available in the supplementary material. We only performed assays on formalin fixed paraffin blocks of biopsies that had at least a 20% tumor infiltration upon review of H&E stain.
2.4. Statistical analysis
The sample size for the overall trial was calculated to be 180 patients, which would provide sufficient statistical power for the primary endpoint, i.e. time to final treatment failure. The biomarker analysis of the first module of the study was an exploratory analysis. We anticipated adequate BMB and BMA harvest from ≥30% of patients for translational endpoint evaluation based on previous experience, which would be sufficient for prospective assessment of the prespecified androgen signaling signature. Descriptive statistics were used. Time to treatment discontinuation of AA from treatment initiation was estimated by the Kaplan-Meier method. We used the Wilcoxon signed-rank test to assess biomarker change and the Wilcoxon rank-sum test to assess treatment duration between samples with and without the candidate prespecified androgen signaling signature. We used the Fisher exact test to assess significance of associations between two categorical variables. Bonferroni correction was used for multiple comparison adjustment. Adjusted p-value cut-off was 0.007 for associations with molecular tissue biomarkers, 0.025 for serum androgens and 0.01 for clinical characteristics. Correlations between blood and BMA androgens by LCMS were assessed by the Spearman method.
3. Results
3.1. Clinical outcomes
Pretreatment characteristics of 170 patients enrolled between March 2011 and February 2015 are depicted in Table 1. Median age was 67 years (range: 45–87). 34 patients (20%) had prior chemotherapy and 105 (62%) anti-androgens. Most patients (101, 72%) had a diagnostic Gleason score ≥8, 23 patients (14%) visceral metastases and 46 (27%) ≥ 20 bone metastases.
Table 1:
Baseline patient and tumor characteristics
| Evaluable patients, n | 170 |
| Median age (range), years | 67 (45–87) |
| Race, n (%) | |
| White | 140 (82) |
| Black/African American | 17 (10) |
| Other | 13 (8) |
| Median ECOG performance status (range) | 1 (0–1) |
| Prior treatments | |
| Median prior hormonal treatment lines (range) | 2 (1–5) |
| Prior anti-androgens, n (%) | 105 (62) |
| Prior chemotherapy, n (%) | 34 (20) |
| Median PSA at baseline (range), ng/mL | 20.7 (0.6 – 1655.4) |
| Gleason score at diagnosis, n (%) | |
| ≤7 | 43 (25) |
| ≥ 8 | 101 (72) |
| Not evaluable | 26 (15) |
| ≥20 Bone Metastases, n (%) | 46 (27) |
| Visceral Metastases, n (%) | 23 (14) |
| Bone marrow infiltration, n (%) | |
| Baseline | 48 (28) |
| Evaluable for biomarker analysis at baseline | 46 (27) |
| Any time point | 53 (31) |
ECOG= Eastern Cooperative Oncology Group; PSA= prostate specific antigen
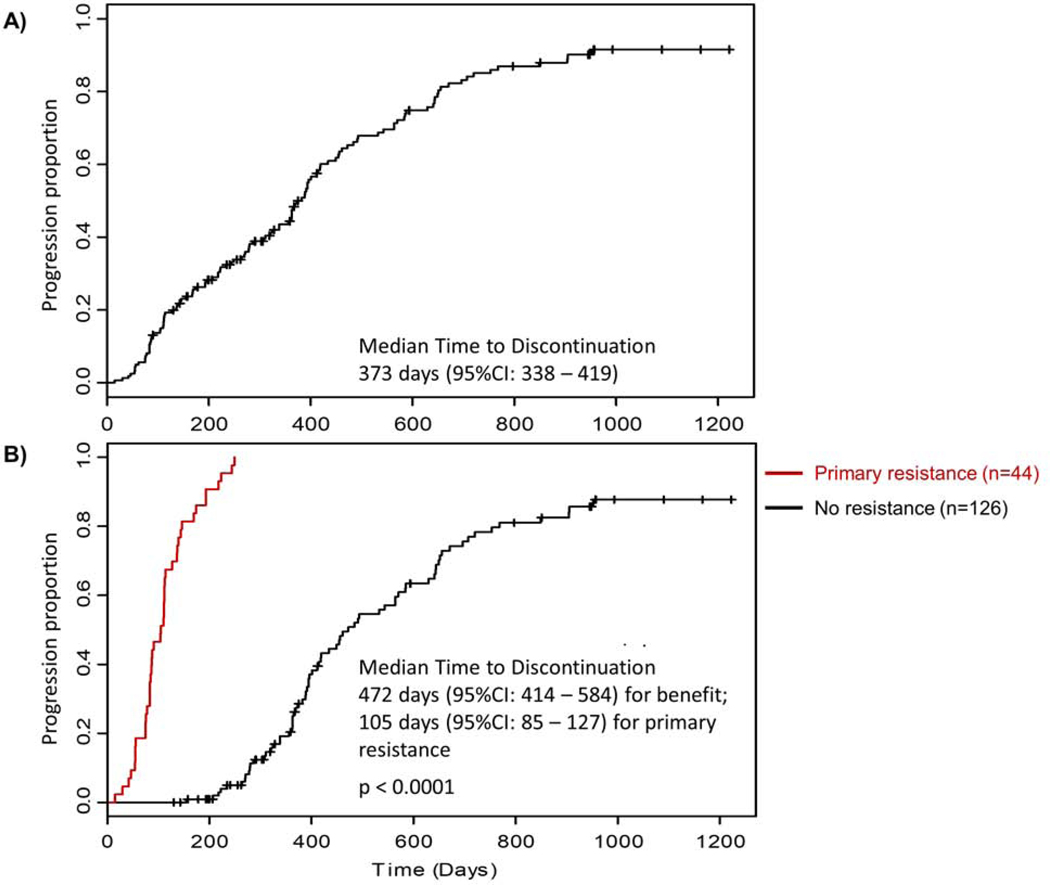
Two patterns of AA response were observed as per our prior experience: primary resistance, i.e. progression within 4 months of drug initiation versus benefit [13]. Forty four patients (26%) exhibited primary resistance. Median time to AA discontinuation was 373 days (95%CI: 338 – 419) (Figure 2a), while for patients with primary resistance 105 days (95%CI: 85–127) and for the remainder 472 days (95%CI: 414 – 584) (Figure 2b).
Figure 2: Time to discontinuation of abiraterone acetate.
A: Time to discontinuation of abiraterone acetate (n=170)
B: Time to discontinuation of abiraterone acetate in patients with primary resistance (n=44) versus no resistance (n=126)
Therapy was well tolerated with most adverse events categorized as grade 1/2 (NCI Common Terminology Criteria for Adverse Events), consistent with reported AA safety. Most patients (123/160, 77%), experienced a maximal PSA decline ≥30%, 66% of the patients ≥50%, and 33% of the patients ≥ 90% (Supplementary Figure S1).
3.2. Biomarker expression and associations with response to AA
Biomarker expression was evaluable in 46 of 48 pretreatment BMB specimens. 21 of those specimens (46%) were obtained from patients with primary resistance to AA.
Testing the androgen signaling signature
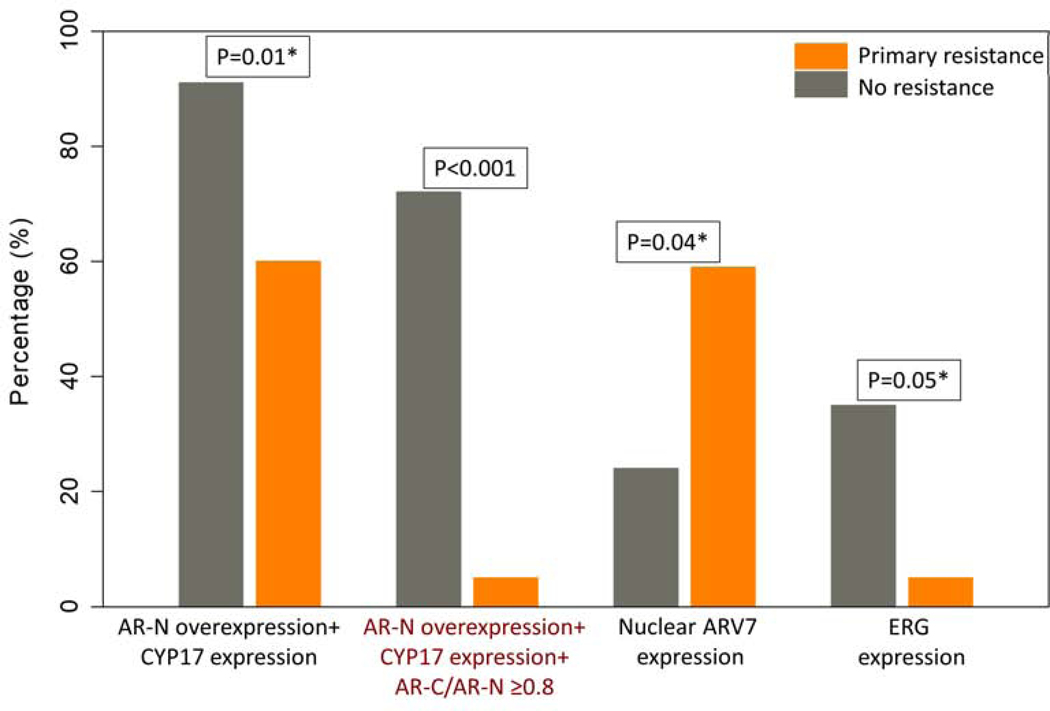
The pretreatment signature (AR-N terminal overexpression with CYP17 expression and a ratio of AR-C terminal / AR-N terminal expression ≥ 0.8) was present in 19 of 25 patients (76%) who exhibited response to AA treatment and 1 of 21 patients (5%) with primary resistance. Presence of the pretreatment androgen signaling signature exhibited significant predictive value regarding clinical benefit from treatment with AA (p <0.001). The full 3-element signature demonstrated clearly higher predictive performance compared to the presence of only 2 out of the 3 signature elements (AR-N terminal overexpression coupled with CYP17 expression) (p=0.01) . (Figure 3, Table 2)
Figure 3: Association of pretreatment tissue biomarkers with outcome.
P values derived from Fischer’s test comparing biomarker(s) presence in patients with primary resistance versus no resistance.
*Not significant after adjustment of multiple comparisons based on Bonferroni correction
Table 2:
Association of molecular tumor markers with outcome
| Tumor Markers, n / total evaluable samples (%) | Primary Resistance | No resistance | P value Fisher’s Test | Bonferroni correction** | Odds Ratio (95% CI) |
|---|---|---|---|---|---|
| Androgen signaling signature* | 1/21 (5) | 19 /25 (76) | < 0.001 | significant | 0.016 (0.002, 0.144) |
| ARV7 expression | 13/21 (62) | 6/25 (24) | 0.04 | non-significant | 5.15 (1.4, 18.36) |
| ERG expression | 1/21 (5) | 9/25 (36) | 0.05 | non-significant | 0.09 (0.01, 0.78) |
| Ki67 >20% | 3/18 (17) | 6/25 (24) | 0.71 | non-significant | 0.63 (0.14, 2.96) |
| GR expression | 8/18 (44) | 12/25 (48) | 0.99 | non-significant | 0.87 (0.26, 2.93) |
| Phospho-Src | 16/21 (76) | 24/25 (96) | 0.08 | non-significant | 0.13 (0.01, 1.25) |
| VEGF expression | 11/12 (92) | 21/22 (95) | 0.99 | non-significant | 0.53 (0.03, 9.20) |
Androgen signalling signature consists of AR-N overexpression + CYP17 expression + AR-C/AR-N ≥0.8
Adjusted p-value cut-off is 0.007 based on Bonferroni correction.
Assessment of further tissue biomarkers
Out of the molecular markers assessed, presence of ERG and nuclear ARV7 were associated with outcome. Presence of nuclear ARV7 was associated with primary resistance to AA (p=0.04). Presence of ERG was associated with response to treatment with AA (p= 0.05). However, both these associations were not significant based on Bonferroni correction for multiple comparisons. (Figure 3, Table 2)
Ki67 expression >20%, as well as GR, phosphor-Src, and VEGF expression were also assessed and neither was associated with outcome (Table 2).
Assessment of blood androgens
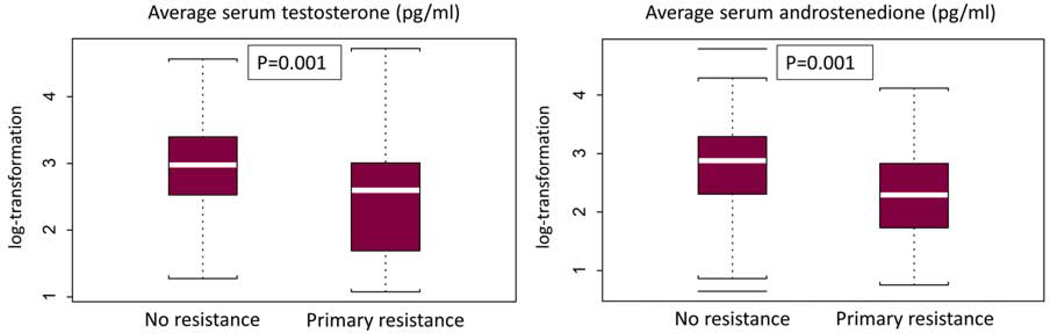
Lower levels of pretreatment androgens (serum testosterone and serum androstenedione) were associated with primary resistance to AA (p=0.001). (Figure 4)
Figure 4: Association of pretreatment serum androgens with outcome.
P values derived from Fischer’s test comparing patients with primary resistance versus no resistance.
3.3. Association of clinical characteristics with response to AA
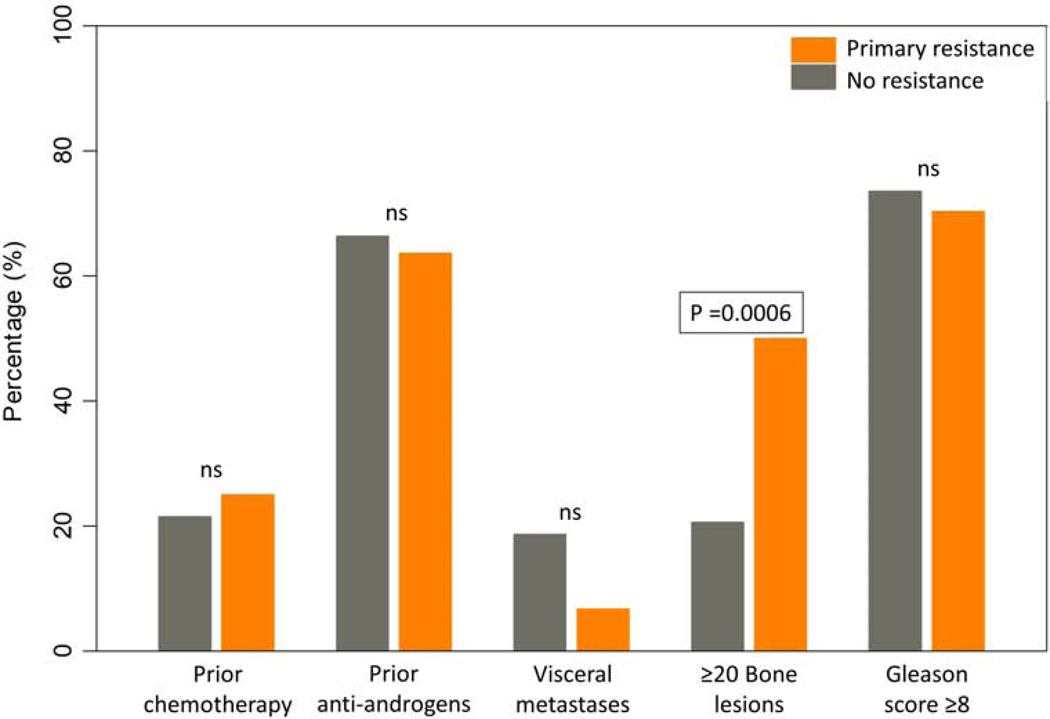
Among examined pre-treatment clinical characteristics, only the presence of 20 or more bone lesions was associated with outcome and more specifically with primary resistance to AA (p=0.0006). No association of response to AA and prior chemotherapy, prior anti-androgens, presence of visceral metastases, or Gleason score ≥ 8 could be detected. (Figure 5)
Figure 5: Association of pretreatment clinical characteristics with outcome.
P values derived from Fischer’s test comparing baseline clinical characteristics in patients with primary resistance versus no resistance. ns = non-significant
3.4. Association of positive androgen signaling signature with clinical characteristics
No significant association was found between the presence of positive pre-treatment androgen signalling signature and the following baseline clinical characteristics: ECOG performance status, prior chemotherapy, presence of visceral metastases, presence of ≥ 20 bone metastases, Gleason score ≥8, age and time to resistance to LHRH analogue. (Table 3 a, b)
Table 3:
Association of positive androgen signaling signature with clinical characteristics A)
| A) | |||
|---|---|---|---|
| Baseline characteristic | Presence of androgen signaling signature * | P value Fisher’s Test | |
| Yes n(%) | No n(%) | ||
| ECOG | |||
| 0 | 6 (24) | 6 (33) | 0.516 |
| 1 | 19 (76) | 12 (67) | |
| Prior Chemotherapy | |||
| Yes | 19 (76) | 13 (72) | 0.999 |
| No | 6 (24) | 5 (28) | |
| Visceral metastases | |||
| Yes | 22 (88) | 15 (83) | 0.683 |
| No | 3 (12) | 3 (17) | |
| ≥20 Bone metastases | |||
| Yes | 12 (48) | 9 (50) | 0.999 |
| No | 13 (52) | 9 (50) | |
| Gleason ≥8 | |||
| Yes | 6 (26) | 10 (56) | 0.105 |
| No | 17 (74) | 8 (44) | |
| B) | |||
| Baseline characteristic | Presence of Androgen signaling signature * | P value Wilcoxon’s Test | |
| Yes | No | ||
| Median (Range) | |||
| Age (years) | 67 (56, 85) | 68 (55, 78) | 0.758 |
|
Time to resistance to LHRH analogue
(months) |
16 (3,91) | 9 (2,128) | 0.095 |
Androgen signalling signature consists of AR-N overexpression + CYP17 expression + AR-C/AR-N ≥0.8.
4. Discussion
The heterogeneous response of mCRPC patients to novel androgen signaling inhibitors has led to a, yet to be, met need for predictors of outcome. Our report on prospective testing of a previously identified, prespecified, candidate androgen signaling signature yielded findings supportive of its predictive value.
AA and enzalutamide initially demonstrated efficacy and were approved internationally for mCRPC treatment. Of note, to date they are mainly used by the community in this setting given reimbursement and other limitations. AA–an androgen biosynthesis inhibitor-and enzalutamide –an AR receptor antagonist-have been shown to improve survival both before and after chemotherapy in this setting in randomized phase III trials [1–4]. In patients with castration resistant disease without metastases, recently reported data from phase III trials showed delayed disease progression with enzalutamide as well as with the newer AR antagonists apalutamide (FDA and EMA approved) and Darolutamide [7–9].
Despite this overall reported benefit, clinical study reports on novel androgen signaling inhibitors’ efficacy are telling of response variability [4, 13–15, 18, 19], with two distinct patterns being identified: approximately one fourth to one third of the patients quickly progress after initiation of treatment deriving no clinical benefit (i.e. primary resistance), while the rest of the patients clearly benefit on these agents. In our analysis, the presence of primary resistance was defined as “composite” progression within four months of treatment initiation. Although this dichotomizing approach may not ideally reflect the different patterns of disease, it enables a well-defined statistical analysis and may serve as a more practical orientation framework for clinical practice.
The observed stage-dependent response to treatments as well as the site-specific preference of progression has led to the speculation that during disease progression prostate cancer evolves into different states and suggests a central role of the microenvironment in bone and prostate –the two preferred sites of progression and recurrence-during this process. In order to further investigate, we previously conducted three translational studies in mCRPC that established the feasibility of serial bone marrow sampling in the clinical setting and identified candidate markers predictive of response to novel androgen signaling inhibitors. Our first study with AA as well as our subsequent study with enzalutamide in bone mCRPC indicated that uniform, intense tumor nuclear AR expression coupled with CYP17 expression were linked to lack of primary resistance to both agents. In our second study, we were the first to report an association between nuclear ARV7 and primary resistance that accounted for some but not all of resistant tumors. In an attempt to account more broadly for other alterations affecting the C terminal AR besides ARV7, we introduced the AR-C terminal/AR-N terminal domain expression ratio. In our subsequent trial with the combination of enzalutamide and AA, we reported that an AR-C terminal/AR-N terminal ratio close to 1 (≥ 0.8), which is indicative of absence of C terminal alterations, enhances the predictive value of the investigated androgen signalling signature. In this study, we performed the initial prespecified testing of this previously discovered 3-element predictive signature with results highly supportive of its predictive value. The next step is to validate these findings in a study, currently underway, selecting for treatment based on signature presence.
Consistent with our initial findings, expression of AR splice variants and specifically AR-V7 have been explored as predictors of resistance to androgen signaling inhibition in multiple subsequent studies [20–23]. Of note, AR-V7 has been linked to worse outcome overall, with a more recent prospective, multicenter trial demonstrating that detection of ARV7 in circulating tumor cells is independently associated with shorter progression free survival (PFS) and overall survival (OS) in patients treated with AA or enzalutamide [24]. This may be more suggestive of a role as a poor prognosticator of outcome, but this remains beyond the scope of this report. Importantly, current assays employed vary in sensitivity and specificity and do not account for a significant number of primary resistant tumors, nor have they attempted to benchmark the liquid biopsy findings against tissue based findings.
We also report a trend for an association between ERG expression and response to therapy with AA. The presence of TMPRSS2-ERG gene fusion, resulting in ERG overexpression, has been previously studied as a potential biomarker of response to prostate cancer treatments with however contradicting results. Patients with tumors harboring an ERG fusion secondary to deletion of 21q22 coupled with an increased copy number of fusion sequences were found to derive greater benefit from AA compared to patients with other classes of ERG rearrangement or absence of ERG fusion [25]. Other reports have been controversial [26, 27].
Mutations in the tumor suppression genes TP53, Rb loss and PTEN loss have also been identified [28, 29] and associated with poor clinical outcomes in advanced prostate cancer. [30, 31] Tumors harboring such genomic alterations typically express an androgen-independent phenotype similar to neuroendocrine prostate cancer. [30] Neuroendocrine prostate cancer differentiation, indicated by markers such as chromogranin and CD56, is characterized by anaplastic features, rendering the cancerous cells capable of escaping hormonal manipulation therapies. [32] These markers of androgen-independent cancer growth and likely primary resistance to androgen signaling inhibition will potentially complement the identified biomarker signature in identifying patients that will derive maximal benefit from androgen signaling inhibition.
We would advocate that the introduction of IHC assays in prostate cancer tissue characterization is long overdue. It is lagging as compared to other solid tumors and this-in conjunction with the more delayed drug development in our field-has likely impacted the delay of therapeutic development as compared to breast cancer and other solid tumors. Immunohistochemistry is a reliable methodology that is inexpensive and universally accessible, i.e. the “low hanging fruit” of molecular characterization. Tissue sampling limitations are with the advent of interventional radiology gradually being overcome. And even though for the purpose of our study characterization was performed on current biopsies, we do anticipate that archival tissue will ultimately be helpful in guiding us towards the appropriate therapeutic strategy.
Even though testing of the candidate androgen signaling signature was prespecified, the study was not powered for this analysis. Tumor infiltration yield was low for patients overall with the sample of evaluable patients being relatively small, indicating the limitation of standard BMB as a tissue acquisition method for routine biomarker testing. Patients with primary resistance were enriched for bone marrow infiltration, which is also in line with the association between extent of tumor volume and outcome. We are currently conducting a validation trial prospectively collecting samples using CT guided biopsy technique and preselecting patients to be treated based on the presence of the candidate signature. Of note, we are also collecting archival baseline tissue to further associate outcomes with diagnostic biopsy findings (NCT03360721).
In conclusion, the initial prespecified testing of a previously identified androgen signaling molecular signature is highly supportive of its predictive value in maximal androgen deprivation strategies in mCRPC. These findings are being validated in a study selecting for treatment based on signature presence.
Supplementary Material
Highlights:
A previously identified pretreatment androgen signaling signature is predictive of response to abiraterone in mCRPC.
We report a trend for an association of ERG expression with benefit and nuclear ARV7 with primary resistance to abiraterone.
Validation of the enhanced signature is currently under way.
Acknowledgments
The authors acknowledge all the participating physicians, research support staff, and patients who participated in this study for their valuable contribution.
Funding
This study received financial support by the Prostate Cancer Foundation. Study drugs were provided by Janssen, Bristol-Myers Squibb Company (BMS), and Pfizer.
Footnotes
Conflict of Interest Statement
M.B., N.S., J.A.W., A.T., A.H., A.A., S.M.T., J.C.A., A.J.Z., P.C., J.W., P.T. and S.W. have no conflict of interest to declare. L.P. has received research funding from Pfizer, Roche/Genentech, Exelixis, and Merck and travel from Merck. J.K. is an employee and owns stocks at Merck. S.S. has consulting/advisory relationship with Valeant, Dendreon, Apricity Health, Janssen, Polaris, Amgen, Bayer, and Exelixis, has received research funding from Janssen, Bristol-Myers Squibb, and AstraZeneca, has received honoraria from Compugen, Apricity Health, Janssen, Dendreon, Polaris, Parker Institute of Cancer Immunotherapy, and Society for Immunotherapy of Cancer, and has ownership interest with Apricity Health. N.T. has consulting/advisory relationship with Bristol-Myers-Squibb and Pfizer, has received research funding from Bristol-Myers-Squibb and honoraria from Bristol-Myers-Squibb and Pfizer, and has participated in scientific advisory committees for Pfizer. J.C.L. has consulting/advisory relationship with Janssen, has received research funding from Bristol-Myers-Squibb, Janssen and Pfizer, has received honoraria from Janssen, and has participated in scientific advisory committees for Pfizer. E.E. has received research grant support, ad board, honoraria and travel from Sanofi, Janssen, Astellas, Tolmar, Bayer.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fizazi K, Scher HI, Molina A et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2012; 13: 983–992. [DOI] [PubMed] [Google Scholar]
- 2.Ryan CJ, Smith MR, Fizazi K et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2015; 16: 152–160. [DOI] [PubMed] [Google Scholar]
- 3.Beer TM, Armstrong AJ, Rathkopf DE et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 2014; 371: 424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scher HI, Fizazi K, Saad F et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012; 367: 1187–1197. [DOI] [PubMed] [Google Scholar]
- 5.Fizazi K, Tran N, Fein L et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med 2017; 377: 352–360. [DOI] [PubMed] [Google Scholar]
- 6.James ND, de Bono JS, Spears MR et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N Engl J Med 2017; 377: 338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fizazi K, Shore N, Tammela TL et al. Darolutamide in Nonmetastatic, Castration-Resistant Prostate Cancer. New England Journal of Medicine 2019; 380: 1235–1246. [DOI] [PubMed] [Google Scholar]
- 8.Smith MR, Saad F, Chowdhury S et al. Apalutamide Treatment and Metastasis-free Survival in Prostate Cancer. N Engl J Med 2018; 378: 1408–1418. [DOI] [PubMed] [Google Scholar]
- 9.Hussain M, Fizazi K, Saad F et al. Enzalutamide in Men with Nonmetastatic, Castration-Resistant Prostate Cancer. N Engl J Med 2018; 378: 2465–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis ID, Martin AJ, Stockler MR et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N Engl J Med 2019. [DOI] [PubMed] [Google Scholar]
- 11.Chi KN, Agarwal N, Bjartell A et al. Apalutamide for Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med 2019. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong AJ, Szmulewitz RZ, Petrylak DP et al. Phase III study of androgen deprivation therapy (ADT) with enzalutamide (ENZA) or placebo (PBO) in metastatic hormone-sensitive prostate cancer (mHSPC): The ARCHES trial. Journal of Clinical Oncology 2019; 37: 687–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Efstathiou E, Titus M, Tsavachidou D et al. Effects of abiraterone acetate on androgen signaling in castrate-resistant prostate cancer in bone. J Clin Oncol 2012; 30: 637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Efstathiou E, Titus M, Wen S et al. Molecular characterization of enzalutamide-treated bone metastatic castration-resistant prostate cancer. Eur Urol 2015; 67: 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Efstathiou E, Titus MA, Wen S et al. Enzalutamide (ENZA) in combination with abiraterone acetate (AA) in bone metastatic castration resistant prostate cancer (mCRPC). Journal of Clinical Oncology 2014; 32: 5000–5000. [Google Scholar]
- 16.Scher HI, Halabi S, Tannock I et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 2008; 26: 1148–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Titus MA, Schell MJ, Lih FB et al. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin Cancer Res 2005; 11: 4653–4657. [DOI] [PubMed] [Google Scholar]
- 18.de Bono JS, Logothetis CJ, Molina A et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011; 364: 1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buttigliero C, Tucci M, Bertaglia V et al. Understanding and overcoming the mechanisms of primary and acquired resistance to abiraterone and enzalutamide in castration resistant prostate cancer. Cancer Treat Rev 2015; 41: 884–892. [DOI] [PubMed] [Google Scholar]
- 20.Antonarakis ES, Lu C, Wang H et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med 2014; 371: 1028–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scher HI, Lu D, Schreiber NA et al. Association of AR-V7 on Circulating Tumor Cells as a Treatment-Specific Biomarker With Outcomes and Survival in Castration-Resistant Prostate Cancer. JAMA Oncol 2016; 2: 1441–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Todenhofer T, Azad A, Stewart C et al. AR-V7 Transcripts in Whole Blood RNA of Patients with Metastatic Castration Resistant Prostate Cancer Correlate with Response to Abiraterone Acetate. J Urol 2017; 197: 135–142. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Wang Z, Tang K et al. Prognostic Value of Androgen Receptor Splice Variant 7 in the Treatment of Castration-resistant Prostate Cancer with Next generation Androgen Receptor Signal Inhibition: A Systematic Review and Meta-analysis. Eur Urol Focus 2018; 4: 529–539. [DOI] [PubMed] [Google Scholar]
- 24.Armstrong AJ, Halabi S, Luo J et al. Prospective Multicenter Validation of Androgen Receptor Splice Variant 7 and Hormone Therapy Resistance in High-Risk Castration-Resistant Prostate Cancer: The PROPHECY Study. J Clin Oncol 2019; 37: 1120–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Attard G, de Bono JS, Logothetis CJ et al. Improvements in Radiographic Progression-Free Survival Stratified by ERG Gene Status in Metastatic Castration-Resistant Prostate Cancer Patients Treated with Abiraterone Acetate. Clin Cancer Res 2015; 21: 1621–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Attard G, Swennenhuis JF, Olmos D et al. Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant prostate cancer. Cancer Res 2009; 69: 2912–2918. [DOI] [PubMed] [Google Scholar]
- 27.Danila DC, Anand A, Sung CC et al. TMPRSS2-ERG status in circulating tumor cells as a predictive biomarker of sensitivity in castration-resistant prostate cancer patients treated with abiraterone acetate. Eur Urol 2011; 60: 897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cancer Genome Atlas Research N. The Molecular Taxonomy of Primary Prostate Cancer. Cell 2015; 163: 1011–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arora K, Barbieri CE. Molecular Subtypes of Prostate Cancer. Curr Oncol Rep 2018; 20: 58. [DOI] [PubMed] [Google Scholar]
- 30.Hamid AA, Gray KP, Shaw G et al. Compound Genomic Alterations of TP53, PTEN, and RB1 Tumor Suppressors in Localized and Metastatic Prostate Cancer. Eur Urol 2019; 76: 89–97. [DOI] [PubMed] [Google Scholar]
- 31.Maughan BL, Guedes LB, Boucher K et al. p53 status in the primary tumor predicts efficacy of subsequent abiraterone and enzalutamide in castration-resistant prostate cancer. Prostate Cancer Prostatic Dis 2018; 21: 260–268. [DOI] [PubMed] [Google Scholar]
- 32.Fine SW. Neuroendocrine tumors of the prostate. Mod Pathol 2018; 31: S122–132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.