Abstract
Background
Patients with inflammatory bowel disease (IBD) are at increased risk for pneumonia, and corticosteroids are reported to amplify this risk. Less is known about the impact of corticosteroid-sparing IBD therapies on pneumonia risk or the efficacy of pneumococcal vaccination in reducing all-cause pneumonia in real-world IBD cohorts.
Methods
We performed a population-based study using an established Veterans Health Administration cohort of 29,957 IBD patients. We identified all patients who developed bacterial pneumonia. Cox survival analysis was used to determine the association of corticosteroids at study entry and as a time-varying covariate, corticosteroid-sparing agents (immunomodulators and antitumor necrosis-alpha [TNF] inhibitors), and pneumococcal vaccination with the development of all-cause pneumonia.
Results
Patients with IBD who received corticosteroids had a greater risk of pneumonia when controlling for age, gender, and comorbidities (hazard ratio [HR] 2.21; 95% confidence interval [CI], 1.90–2.57 for prior use; HR = 3.42; 95% CI, 2.92–4.01 for use during follow-up). Anti-TNF inhibitors (HR 1.52; 95% CI, 1.02–2.26), but not immunomodulators (HR 0.91; 95% CI, 0.77–1.07), were associated with a small increase in pneumonia. A history of pneumonia was strongly associated with subsequent pneumonia (HR = 4.41; 95% CI, 3.70–5.27). Less than 15% of patients were vaccinated against pneumococcus, and this was not associated with a reduced risk of pneumonia (HR = 1.02; 95% CI, 0.80–1.30) in this cohort.
Conclusion
In a large US cohort, corticosteroids were confirmed to increase pneumonia risk. Tumor necrosis-alpha inhibitors were associated with a smaller increase in the risk of pneumonia. Surprisingly, pneumococcal vaccination did not reduce all-cause pneumonia in this population, though few patients were vaccinated.
Keywords: inflammatory bowel disease, pneumonia, vaccine
INTRODUCTION
Inflammatory bowel disease (IBD) has a prevalence of over 200 per 100,000 and is expected to affect over 2 million people in the United States by 2025.1–3 Infections are common in patients with IBD and are linked to morbidity, increased hospitalizations, and higher health care costs.4, 5 Several studies indicate that patients with IBD are at increased risk for pneumonia, particularly in the setting of corticosteroid use.6–9 The impact of immunomodulators and antitumor necrosis alpha (anti-TNF) inhibitors on pneumonia risk, however, is less clear. Some studies have shown an increased risk of infection with anti-TNF inhibitors,6 but others have not.8, 9 A study using administrative claims data found anti-TNF inhibitors was associated with increased risk of pneumonia.6 In contrast, both a prospective registry of Crohn’s disease (CD) patients and a national database including patients with CD and ulcerative colitis (UC) found anti-TNF inhibitors were not associated with an increased risk of infection.8, 9
Current IBD quality measures recommend that all patients with IBD undergo vaccination against pneumococcal pneumonia with administration of the pneumococcal conjugate vaccine (PCV13) followed at least 8 weeks later by the pneumococcal polysaccharide vaccine (PPSV23), followed by a second PPSV23 dose 5 years later and a third dose at age 65.10, 11 Studies, however, have suggested the immune response to pneumococcal vaccination is blunted in IBD patients on immunosuppressants.12, 13 Our aim was to examine the association between corticosteroids and corticosteroid-sparing agents on the risk of all-cause pneumonia in patients with IBD and to explore the real-world effectiveness of pneumococcal vaccination in reducing all-cause pneumonia incidence in a large US population.
METHODS
Study Population
We conducted a retrospective population-based study using an established longitudinal cohort of Veterans with IBD.14 Patients were identified using previously validated algorithms based on a combination of inpatient and outpatient International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for CD (555.x), and UC (556.x). Patients were selected for inclusion if they had 2 or more of these ICD-9-CM codes during at least 2 clinical encounters between 2002 and 2009, with at least 1 of these encounters being an outpatient visit. This approach has a positive predictive value for CD of 0.84 and a positive predictive value for UC of 0.91 in the Veterans Health Administration (VHA).15 Veterans were classified as CD if all ICD-9-CM codes were 555.x or as UC if all codes were 556.x, and the remaining were classified as indeterminate colitis (IC). The date of the first encounter with an IBD ICD-9-CM code was considered the “IBD index date” for study purposes. Patients were identified with an IBD diagnosis between 2002 and 2009 and monitored for outcomes until the end of 2011 for at least 2 years of follow-up.
Variables
Baseline patient demographics were collected at the IBD index date along with comorbidities, including previous bacterial pneumonia from the year before IBD index date through the year after the IBD index date. We identified all prescriptions filled for corticosteroids during the year before the IBD index date and the year after the IBD index date. Any patient that had an ICD-9-CM code for chronic obstructive pulmonary disease, asthma, rheumatoid arthritis, lupus, dermatitis, bronchitis, sinusitis, rhinitis, polymyalgia rheumatic, autoimmune hepatitis, or cluster headaches within 7 days of a corticosteroid prescription was defined as having corticosteroids for a non-IBD condition, and those fills were excluded from further analysis. We excluded corticosteroid use because our focus was on the decision IBD providers make in treating IBD flares. Any patient that was prescribed a corticosteroid-sparing agent (immunomodulator and/or anti-TNF inhibitor) in the year after the IBD index date was defined as having escalated therapy. Information on whether patients had a prior PPSV23 vaccination was gathered during the year before and the year after IBD index date (CPT codes 90732; ICD-9-CM code v03.82). Based on the date of approval of pneumococcal vaccinations in the United States, nearly all pneumococcal vaccinations were PPSV23. We used the Charlson comorbidity index to identify comorbidities.16
Outcomes
The primary outcome was new diagnosis of bacterial pneumonia, identified as any encounter with an ICD-9-CM code for bacterial pneumonia (481, 482–483, 485, 486), which has been previously validated in administrative data.17 Pneumonia was assessed during our follow-up time period, from 1 year after the IBD index date until the end of the study period (through 2011). We used this timeframe to allow for inclusion of comorbidities around IBD diagnosis (1 year prior and 1 year after) and IBD treatment that may have occurred soon after diagnosis. We used the first occurrence of pneumonia diagnosis during the follow-up period in the event that patients had more than 1 diagnosis during follow-up.
Statistical Analysis
Differences in baseline characteristics between those who developed pneumonia and those that did not were compared using the Pearson χ 2 test for categorical variables and independent samples t test for continuous variables. For comparisons between categorical variables with small sample sizes (i.e., cell counts <5), the Fisher exact test was used. Cox survival models were used to model the time to pneumonia, adjusting for age, male gender, and Charlson comorbidity index. We included a time-varying covariate for corticosteroid use during follow-up. Patients were censored when they had a pneumonia diagnosis, died, or when the study period ended. Since vaccines may be more likely to be provided to patients who are older and sicker, we assessed 2-way interactions for vaccine administration with each of the other predictors (IBD diagnosis type, prior pneumonia, escalation medication, prior steroid use, post steroid use, age, male gender, and Charlson comorbidity). We also examined interactions for escalation medication and steroid use. Interactions were kept in the final model if there was a significant interaction effect (p < 0.05) and/or if inclusion of the interaction modified the main effects for other predictors in the model. We also conducted a sensitivity analysis that included immunomodulators and anti-TNFs as separate predictors.
RESULTS
We identified 29,957 patients with IBD; 35% had CD, 54% had UC, and 11% were classified as having indeterminate colitis (Table 1). A majority were white (69.2%) and male (93.5%) as is reflective of the VHA population. The percentage of patients exposed to corticosteroids at baseline was 18.8%, and 12.6% were escalated to a corticosteroid-sparing agent. Patients with indeterminate colitis had higher rates of baseline steroid use (32.7%) and escalation medications (22.9%) (Supplement Tables 1 and 2). One fifth (20.3%) of patients had corticosteroid use during follow-up; these rates were higher for patients who developed pneumonia (33.5% vs 19.5%) (Table 2). Patients were followed for an average of 1749 days (standard deviation = 908), and 5.3% developed pneumonia during this follow-up period. Similar rates of pneumonia were seen in patients with CD and UC (4.9% in each), whereas 8.1% of patients with IC developed pneumonia (Supplement Table 1).
TABLE 1.
Patient Characteristics at Baseline for Patients with Ulcerative Colitis and Crohn’s Disease
| Total | Pneumonia after IBD Diagnosis | |||
|---|---|---|---|---|
| No | Yes | P | ||
| Patients, N (%) | 29,957 (100%) | 28,376 (94.7%) | 1581 (5.3%) | |
| IBD Type | ||||
| CD | 10,433 (34.8%) | 9919 (35.0%) | 514 (32.5%) | <0.001 |
| UC | 16,179 (54.0%) | 15,382 (54.2%) | 797 (50.4%) | |
| IC | 3345 (11.2%) | 3075 (10.8%) | 270 (17.1%) | |
| Age, years Mean (SD) | 60.2 (15.3) | 60.0 (15.4) | 63.1 (13.1) | <0.001 |
| Male, N (%) | 28,011 (93.5%) | 26,526 (93.5%) | 1485 (93.9%) | 0.480 |
| Race, N (%) | ||||
| Caucasian | 20,725 (69.2%) | 19,477 (68.6%) | 1248 (78.9%) | <0.001 |
| African American | 2075 (6.9%) | 1967 (6.9%) | 108 (6.8%) | |
| Other | 484 (1.6%) | 461 (1.6%) | 23 (1.5%) | |
| Unknown/Missing | 6673 (22.3%) | 6471 (22.8%) | 202 (12.8%) | |
| Prior Pneumoniaa | 601 (2.0%) | 430 (1.5%) | 171 (10.8%) | <0.001 |
| Vaccinea | 3981 (13.3%) | 3694 (13.0%) | 287 (18.2%) | <0.001 |
| Prior Steroid Usea | 5629 (18.8%) | 5071 (17.9%) | 558 (35.3%) | <0.001 |
| Escalation Medicationa | 3787 (12.6%) | 3533 (12.5%) | 254 (16.1%) | <0.001 |
| Escalation Medication Type | ||||
| No Escalation Medication | 31,869 (88.5%) | 25,717 (90.6%) | 1385 (87.6%) | <0.001 |
| Any Anti-TNF | 480 (1.3%) | 285 (1.0%) | 29 (1.8%) | |
| Immunomodulator | 3679 (10.2%) | 2374 (8.4%) | 167 (10.6%) | |
| CCI ≥ 1a | 10,791 (36.0%) | 9773 (34.4%) | 1018 (64.4%) | <0.001 |
Abbreviations: CCI, Charlson Comorbidity Index
a Timeframe for defining measures include 1 year prior and 1 year after IBD diagnosis.
TABLE 2.
Patient Follow-up Information
| Total | Pneumonia after IBD Diagnosis | ||
|---|---|---|---|
| No | Yes | ||
| Patients, N (%) | 29,957 (100%) | 28,376 (94.7%) | 1581 (5.3%) |
| Follow-up, days Mean (SD) | 1749 (908) | 1790 (897) | 1018 (772) |
| Steroid at baseline, N (%) | 5629 (18.8%) | 5071 (17.9%) | 558 (35.3%) |
| Steroid during follow-upa, N (%) | 6071 (20.3%) | 5541 (19.5%) | 530 (33.5%) |
| Steroid at baseline only, N (%) | 2687 (9.3%) | 2424 (8.5%) | 263 (16.6%) |
| Steroid during follow-up onlya, N (%) | 3129 (10.4%) | 2894 (10.2%) | 235 (14.9%) |
| Steroid at baseline and during follow-up,a N (%) | 2942 (9.8%) | 2647 (9.3%) | 295 (18.7%) |
| No steroid use, N (%) | 21,199 (70.8%) | 20,411 (71.9%) | 788 (49.8%) |
aIncludes steroid use during follow-up and before censoring.
Risk Factors for Pneumonia in IBD Patients
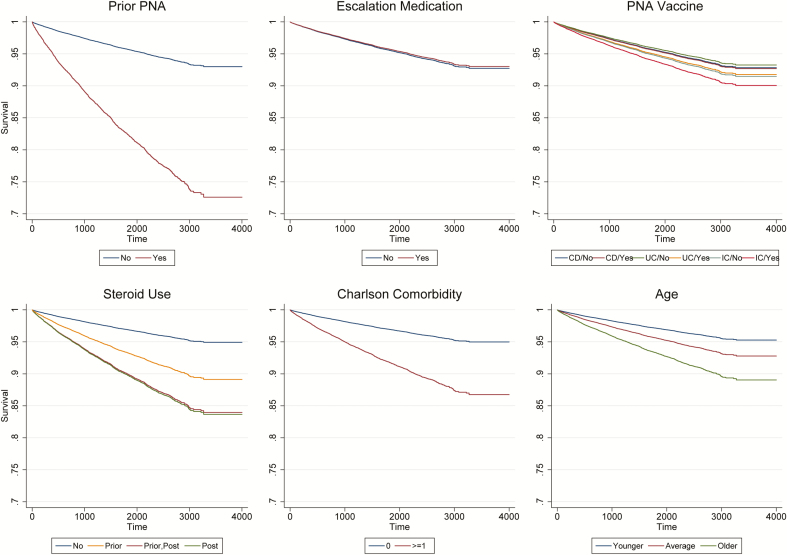
Patients who developed pneumonia were older with more comorbidities and were more likely to have had a history of pneumonia than those who did not develop pneumonia (Table 1). They were also more likely to have been exposed to corticosteroids and the pneumonia vaccine and to be diagnosed with IC compared with CD and UC. Table 3 shows the Cox survival model results for time to pneumonia, and Figure 1 displays the survival curves. After controlling for age, gender, and comorbidities, patients who had at least 1 prescription fill for corticosteroids before follow-up had a greater risk of pneumonia (hazard ratio [HR] 2.21; 95% CI, 1.90–2.57). Similarly, steroid use during follow-up was associated with a greater risk of pneumonia (HR 3.42; 95% CI, 2.92–4.01). The interaction effect for prior steroid use and steroid use during follow-up use was also significant (HR 0.44; 95% CI, 0.35–0.56), indicating that continued steroid use was associated with an additive effect on risk of pneumonia compared with steroid use only during the baseline period (Fig. 1). In contrast, patients that were escalated to a corticosteroid-sparing agent did not have an increased risk of pneumonia (HR 0.96; 95% CI, 0.83–1.11).
TABLE 3.
Predictors of Time to Pneumonia in IBD patients
| HR | 95% CI | P | |
|---|---|---|---|
| Prior pneumonia | 4.41 | (3.70, 5.27) | <0.001 |
| Escalation medication | 0.96 | (0.83, 1.11) | 0.575 |
| Vaccine | 1.02 | (0.80, 1.30) | 0.864 |
| UC (vs CD) | 0.94 | (0.83, 1.06) | 0.311 |
| IC (vs CD) | 1.20 | (1.02, 1.41) | 0.031 |
| Vaccine X IBD type | |||
| Vaccine X UC | 1.21 | (0.89, 1.63) | 0.220 |
| Vaccine X IC | 1.15 | (0.77, 1.71) | 0.490 |
| Prior steroid use | 2.21 | (1.90, 2.57) | <0.001 |
| Post-steroid use (tvc) | 3.42 | (2.92, 4.01) | <0.001 |
| Prior steroid X post-steroid (tvc) use | 0.44 | (0.35, 0.56) | <0.001 |
| Age (mean-centered) | 1.01 | (1.01, 1.02) | <0.001 |
| Male | 0.89 | (0.72, 1.10) | 0.270 |
| CCI ≥ 1 | 2.77 | (2.48, 3.09) | <0.001 |
Cox survival model was used to predict time to any pneumonia event. Patients were censored when they had an event, when they died, or when the study period ended. Timeframe for defining predictors included 1 year prior and 1 year after IBD diagnosis. Outcomes were defined, and follow-up time started accruing 1 year after IBD diagnosis until the end of the study period. Post-steroid use was included as a time-varying covariate.
FIGURE 1.
Survival curves for predictors of time to pneumonia in IBD patients. Significant predictors included prior pneumonia (PNA), IC (vs CD), prior and post-steroid use (tvc), age (mean-centered), and Charlson comorbidity. Escalation medication and vaccine were not significant.
Patients who had prior pneumonia were more than 4 times as likely to have a subsequent pneumonia diagnosis (HR 4.41; 95% CI, 3.70–5.27) (Table 3). Compared with CD patients, those with IC had a higher risk of pneumonia (HR 1.20; 95% CI, 1.02–1.41). Older patients (HR 1.01; 95% CI, 1.01–1.02) and those with more comorbidities (HR 2.77; 95% CI, 2.48–3.09) also had a higher risk of developing pneumonia (Table 3). Receipt of the pneumonia vaccine, however, was not associated with a lower risk of pneumonia (HR 1.02; 95% CI, 0.80–1.30) (Table 3).
The sensitivity analysis including separate predictors for anti-TNFs and immunomodulators revealed a significant effect for any anti-TNF use (Supplement Table 4). Patients who received anti-TNFs had a higher risk of developing pneumonia compared with those who did not receive a corticosteroid-sparing medication (HR 1.52; 95% CI, 1.02–2.26). The remaining results were consistent with the full model shown in Table 3. The results within each IBD diagnosis type are shown in Supplement Table 3, and survival curves are shown in Supplement Figures 1–3.
Association of Vaccination With Subsequent Pneumonia
Less than 15% of patients received the 23-valent pneumococcal vaccine (Table 1). Interestingly, unadjusted results revealed that patients who developed pneumonia were more likely to have been vaccinated against pneumococcus (Table 1). After controlling for comorbidities, age, and medications, having received a pneumonia vaccine was not associated with a reduced risk of pneumonia (HR 1.02; 95% CI, 0.80–1.30) (Table 3). The interaction effect for vaccine and IBD subtype (CD, UC, IC) was not significant. However, before including the interaction, vaccination was associated with a higher risk of pneumonia (HR 1.15; 95% CI, 1.01–1.32) (Supplement Table 5). It appears this effect was due to UC patients; results from the separate models within each IBD type (Supplement Table 3) showed that the vaccine was associated with a higher risk of pneumonia for UC patients only (HR 1.21; 95% CI, 1.01–1.46). This effect is diminished after controlling for the interaction between vaccination and IBD subtype (Table 3).
We tested for interactions between vaccine and all other predictors (prior pneumonia, escalation medication, prior steroid use, post-steroid use, age, male gender, and Charlson Comorbidity) and between escalation medication and steroid use. The remaining interactions for vaccine, other predictors, escalation medication, and steroid use were not significant, did not change the hazard ratios for the main effects, and thus were not included in the final model shown in Table 3.
DISCUSSION
In a large US cohort of Veterans, corticosteroids were associated with an increased risk of all-cause bacterial pneumonia. Although immunomodulators and anti-TNFs together were not associated with increased risk, an additional sensitivity analysis found that anti-TNF use was associated with greater risk of pneumonia, whereas immunomodulators were not. Previous pneumonia was the greatest risk factor for subsequent pneumonia. Pneumococcal vaccination within 1 year before or after IBD diagnosis was not protective against all-cause pneumonia in this population.
Our findings are consistent with previous studies that have found a marked increased risk of infection with systemic corticosteroids.6, 8, 18 We also found that patients who were escalated to an anti-TNF were associated with an increased risk of pneumonia but not to the same magnitude as corticosteroid use. Escalating to an immunomodulator was not associated with an increased risk of pneumonia. Other studies have found an increased risk of infection with anti-TNF inhibitors,8 particularly with concomitant immunomodulators.18 Some studies, however, suggest some of the increased risk of infection with anti-TNF inhibitors may be attributed to disease activity.8, 18
Our finding that pneumococcal vaccination was not associated with a decreased risk for all-cause pneumonia was surprising. However, several studies have suggested a blunted immune response to pneumococcal vaccines in patients with IBD.12, 13 In particular, combination therapy with an anti-TNF inhibitor and immunomodulator decreases immune response.12, 13 Similar findings have been found with vaccination against influenza19–23 and in other populations on immunosuppressants.24 Our finding that pneumococcal vaccination was not protective suggests that the blunted response to vaccination observed in these studies may have real-world consequences. It could be that we captured vaccination around the time patients were diagnosed with IBD, and many were being treated with immunosuppressant medications. It is also possible that patients were vaccinated before the dates we examined. With less than 15% of patients being vaccinated, it could be that too few patients were vaccinated for us to observe a difference. The mean follow-up was less than 5 years, which may not have been long enough to show an effect. Another possible explanation is that patients were developing pneumonia caused by organisms other than pneumococcus. Indeed, other studies have shown pneumococcal vaccination is protective against pneumococcal bacteremia but not all-cause pneumonia.25, 26 Further studies could examine ways to increase the effectiveness, perhaps by vaccinating early on in the disease course or focusing on patients with an increased risk of developing pneumonia. In our study, receiving corticosteroids and a history of pneumonia were risk factors for pneumonia.
Our finding that UC patients who were vaccinated had an increased risk of pneumonia should be interpreted within the following context: when the observation was carried out for all IBD patients, the effect for vaccination was not significant. Prior publications also provide insight. In a study of older adults, pneumococcal polysaccharide vaccination reduced pneumococcal bacteremia but not risk of hospitalization for pneumonia or outpatient pneumonia.25 In a different immune suppressed population (HIV), pneumococcal vaccine was not found to decrease pneumococcal disease (invasive, serotype specific, or all). In fact, in this study, rates of all-cause pneumonia were actually increased in the vaccinated population.27 The World Health Organization (WHO) and recent meta-analyses also indicate that the real-world data supporting the efficacy and effectiveness of PPV23 in preventing community acquired pneumonia in the general population or immunocompromised subjects are inconsistent.28, 29 The WHO position statement indicates that randomized controlled trials (RCTs) indicated PPV23 is protective against invasive pneumococcal disease (IPD) and all-cause pneumonia in healthy young adults (50–80% effective) and to a lesser degree in those older than 65 years of age. However, they acknowledge that RCTs do not demonstrate efficacy against IPD or all-cause pneumonia in immunocompromised individuals regardless of age.29 Another potential explanation for the association is that patients with more severe IBD or more comorbidities were more likely to be both vaccinated and to develop pneumonia. We used the Charlson comorbidity index to control for comorbid conditions. Controling for corticosteroids and corticosteroid-sparing agents helped control for IBD severity, but we lacked endoscopic, imaging, or laboratory data to more precisely control for disease activity and severity. It is important to mention that this study period precedes standard usage of the more effective PCV13 vaccine.
We found that the biggest risk factor for developing pneumonia was a history of pneumonia. This raises the question as to whether certain patients with an IBD diagnosis are also predisposed to developing bacterial pneumonia. Several lines of evidence support this possibility. Epithelial-related immunity is important to both IBD and pneumonia and many of the IBD susceptibility genes relate to microbial immune response. In support of this theory, one recent study demonstrated that IBD patients had an increased risk of pneumonia up to 5 years before IBD diagnosis.7 Similar to the gut, altered pulmonary epithelial barrier function has also been reported in IBD patients.30, 31 Finally, gut microbiota, which have several important roles in IBD pathophysiology, also appear to play a protective role against pneumococcus32 and influence response to vaccination.33 Together, these observations suggest several potential areas of mechanistic interaction between IBD and pneumonia. Given pneumonia is a major cause for mortality in IBD patients, further study in this relationship is warranted.5
The strengths of our study include the use of a large US-based data set with integrated inpatient, outpatient, and pharmacy data. This allowed us to examine the effects of medications on the risk of pneumonia. Additionally, we utilized an established well-described IBD cohort.15
On the other hand, there are several limitations to our study. Similar to other administrative databases, there is a risk of misclassification, but we used a case-finding algorithm that has been validated in a VA data set.15 The VA population is mostly white males, which limits the generalizability of our findings. Although the VA has a well-integrated electronic medical record system, we would not be able to capture any treatment patients received for IBD or pneumonia outside the VA system. Given the fragmented nature of the US health care system, the VA system is one of the few where most inpatient and outpatient data are available. Thus, we believe this is one of the few data sets in the United States that could address this question with such a large population.
We chose to include ICD-9-CM codes for bacterial pneumonia by any organism, rather than only pneumococcal pneumonia because microbiologic diagnosis is often not achieved in clinical practice.34 It is possible that if we had culture data, we may have observed that pneumococcal vaccination decreased the risk of pneumococcal pneumonia strains in the 23 valent vaccine. However, there were only 66 cases with an ICD-9-CM code for pneumococcal pneumonia in our data set. Given that pneumococcus remains a common cause of bacterial pneumonia,35, 36 we likely would have missed many cases if we only used this ICD-9-CM code. Further research on the microbiology of pneumonia in IBD patients would be helpful. It could be that organisms other than pneumococcus are responsible for a majority of pneumonia cases in IBD patients. Indeed, recent studies have suggested that although pneumococcus remains an important cause of bacterial pneumonia, it no longer causes an overwhelming majority of cases in developed countries.35, 36 The pneumococcal vaccine could be effective in reducing pneumococcal pneumonia but not in all-cause pneumonia.
In summary, we found that corticosteroids are associated with an increased risk of pneumonia in IBD patients. Anti-TNF inhibitors were also associated with an increased risk of pneumonia but not to the same magnitude as corticosteroids. Immunomodulators were not associated with an increased risk of pneumonia. This may have caveats to treatment decisions, particularly in the setting of concerns for the risk of infections like pneumonia. Despite recommendations for vaccinating all IBD patients against pneumococcus, we found that few patients were vaccinated with the PPSV23, and this was not associated with a decreased risk of all-cause pneumonia in this population. Future studies are needed to determine if the vaccination with PCV13 that became available after the study period decreases all-cause pneumonia. Further research into the pathophysiology of pneumonia in IBD patients may yield insights into why pneumococcal vaccination may not decrease all-cause pneumonia in IBD patients.
Supplementary Material
Supported by: The research reported here was supported in part by career development grant CDA 11–217 and merit review award IIR 16–024 from the US Department of Veterans Affairs Health Services Research and Development Service (AKW),VA HSR&D Center for Innovations in Quality, Effectiveness and Safety (#CIN 13–413), at the Michael E. DeBakey VA Medical Center, Houston, TX (JKH). Philanthropic support from Lawrence C. Pakula IBD Research Innovators Fund at Washington University (MAC) and the Givin’ It All For Guts Foundation (MAC).
Conflicts of interest: MAC is on the speakers bureau or consulting for Pfizer, UCB, AbbVie, and Takeda. LAF is involved with clinical trials for Takeda Pharmaceutical. MHG, WLW, RWS, PH, SCM, JKH, SMG, SAC, and AKW have no financial conflicts of interest to disclose.
REFERENCES
- 1. Loftus CG, Loftus EV Jr, Harmsen WS, et al. . Update on the incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota, 1940-2000. Inflamm Bowel Dis. 2007;13:254–261. [DOI] [PubMed] [Google Scholar]
- 2. Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. . The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5:1424–1429. [DOI] [PubMed] [Google Scholar]
- 3. Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology. 2017;152:313–321.e2. [DOI] [PubMed] [Google Scholar]
- 4. Hutfless SM, Weng X, Liu L, et al. . Mortality by medication use among patients with inflammatory bowel disease, 1996-2003. Gastroenterology. 2007;133:1779–1786. [DOI] [PubMed] [Google Scholar]
- 5. Ananthakrishnan AN, McGinley EL. Infection-related hospitalizations are associated with increased mortality in patients with inflammatory bowel diseases. J Crohns Colitis. 2013;7:107–112. [DOI] [PubMed] [Google Scholar]
- 6. Long MD, Martin C, Sandler RS, et al. . Increased risk of pneumonia among patients with inflammatory bowel disease. Am J Gastroenterol. 2013;108:240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kantsø B, Simonsen J, Hoffmann S, et al. . Inflammatory bowel disease patients are at increased risk of invasive pneumococcal disease: a nationwide Danish cohort study 1977-2013. Am J Gastroenterol. 2015;110:1582–1587. [DOI] [PubMed] [Google Scholar]
- 8. Lichtenstein GR, Feagan BG, Cohen RD, et al. . Serious infections and mortality in association with therapies for Crohn’s disease: TREAT registry. Clin Gastroenterol Hepatol. 2006;4:621–630. [DOI] [PubMed] [Google Scholar]
- 9. Schneeweiss S, Korzenik J, Solomon DH, et al. . Infliximab and other immunomodulating drugs in patients with inflammatory bowel disease and the risk of serious bacterial infections. Aliment Pharmacol Ther. 2009;30:253–264. [DOI] [PubMed] [Google Scholar]
- 10. Reich J, Wasan S, Farraye FA. Vaccinating patients with inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2016;12:540–546. [PMC free article] [PubMed] [Google Scholar]
- 11. Farraye FA, Melmed GY, Lichtenstein GR, et al. . ACG clinical guideline: preventive care in inflammatory bowel disease. Am J Gastroenterol. 2017;112:241–258. [DOI] [PubMed] [Google Scholar]
- 12. Melmed GY, Agarwal N, Frenck RW, et al. . Immunosuppression impairs response to pneumococcal polysaccharide vaccination in patients with inflammatory bowel disease. Am J Gastroenterol. 2010;105:148–154. [DOI] [PubMed] [Google Scholar]
- 13. Fiorino G, Peyrin-Biroulet L, Naccarato P, et al. . Effects of immunosuppression on immune response to pneumococcal vaccine in inflammatory bowel disease: a prospective study. Inflamm Bowel Dis. 2012;18:1042–1047. [DOI] [PubMed] [Google Scholar]
- 14. Waljee AK, Wiitala WL, Govani S, et al. . Corticosteroid use and complications in a US inflammatory bowel disease cohort. Plos One. 2016;11:e0158017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hou JK, Tan M, Stidham RW, et al. . Accuracy of diagnostic codes for identifying patients with ulcerative colitis and Crohn’s disease in the Veterans Affairs Health Care System. Dig Dis Sci. 2014;59:2406–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Charlson ME, Pompei P, Ales KL, et al. . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 17. Wiese AD, Griffin MR, Stein CM, et al. . Validation of discharge diagnosis codes to identify serious infections among middle age and older adults. BMJ Open. 2018;8:e020857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Osterman MT, Sandborn WJ, Colombel JF, et al. . Crohn’s disease activity and concomitant immunosuppressants affect the risk of serious and opportunistic infections in patients treated with adalimumab. Am J Gastroenterol. 2016;111:1806–1815. [DOI] [PubMed] [Google Scholar]
- 19. Mamula P, Markowitz JE, Piccoli DA, et al. . Immune response to influenza vaccine in pediatric patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2007;5:851–856. [DOI] [PubMed] [Google Scholar]
- 20. Cullen G, Bader C, Korzenik JR, et al. . Serological response to the 2009 H1N1 influenza vaccination in patients with inflammatory bowel disease. Gut. 2012;61:385–391. [DOI] [PubMed] [Google Scholar]
- 21. deBruyn JC, Hilsden R, Fonseca K, et al. . Immunogenicity and safety of influenza vaccination in children with inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:25–33. [DOI] [PubMed] [Google Scholar]
- 22. Andrisani G, Frasca D, Romero M, et al. . Immune response to influenza A/H1N1 vaccine in inflammatory bowel disease patients treated with anti TNF-α agents: effects of combined therapy with immunosuppressants. J Crohns Colitis. 2013;7:301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hagihara Y, Ohfuji S, Watanabe K, et al. . Infliximab and/or immunomodulators inhibit immune responses to trivalent influenza vaccination in adults with inflammatory bowel disease. J Crohns Colitis. 2014;8:223–233. [DOI] [PubMed] [Google Scholar]
- 24. Agarwal N, Ollington K, Kaneshiro M, et al. . Are immunosuppressive medications associated with decreased responses to routine immunizations? A systematic review. Vaccine. 2012;30:1413–1424. [DOI] [PubMed] [Google Scholar]
- 25. Jackson LA, Neuzil KM, Yu O, et al. ; Vaccine Safety Datalink Effectiveness of pneumococcal polysaccharide vaccine in older adults. N Engl J Med. 2003;348:1747–1755. [DOI] [PubMed] [Google Scholar]
- 26. Hechter RC, Chao C, Jacobsen SJ, et al. . Clinical effectiveness of pneumococcal polysaccharide vaccine in men: California Men’s Health Study. Vaccine. 2012;30:5625–5630. [DOI] [PubMed] [Google Scholar]
- 27. French N, Nakiyingi J, Carpenter LM, et al. . 23-valent pneumococcal polysaccharide vaccine in HIV-1-infected Ugandan adults: double-blind, randomised and placebo controlled trial. Lancet. 2000;355:2106–2111. [DOI] [PubMed] [Google Scholar]
- 28. Tin Tin Htar M, Stuurman AL, Ferreira G, et al. . Effectiveness of pneumococcal vaccines in preventing pneumonia in adults, a systematic review and meta-analyses of observational studies. Plos One. 2017;12:e0177985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. World Health Organization. 23-valent pneumococcal polysaccharide vaccine. WHO position paper. Wkly Epidemiol Rec 2008;83:373–384. [PubMed] [Google Scholar]
- 30. Adenis A, Colombel JF, Lecouffe P, et al. . Increased pulmonary and intestinal permeability in Crohn’s disease. Gut. 1992;33:678–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Robertson DA, Taylor N, Sidhu H, et al. . Pulmonary permeability in coeliac disease and inflammatory bowel disease. Digestion. 1989;42:98–103. [DOI] [PubMed] [Google Scholar]
- 32. Schuijt TJ, Lankelma JM, Scicluna BP, et al. . The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut. 2016;65:575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zimmermann P, Curtis N. The influence of the intestinal microbiome on vaccine responses. Vaccine. 2018;36:4433–4439. [DOI] [PubMed] [Google Scholar]
- 34. Bartlett JG. Diagnostic tests for agents of community-acquired pneumonia. Clin Infect Dis. 2011;52(Suppl 4):S296–S304. [DOI] [PubMed] [Google Scholar]
- 35. Musher DM, Abers MS, Bartlett JG. Evolving understanding of the causes of pneumonia in adults, with special attention to the role of pneumococcus. Clin Infect Dis. 2017;65:1736–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jain S, Self WH, Wunderink RG, et al. ; CDC EPIC Study Team Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373:415–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.