Figure 8.
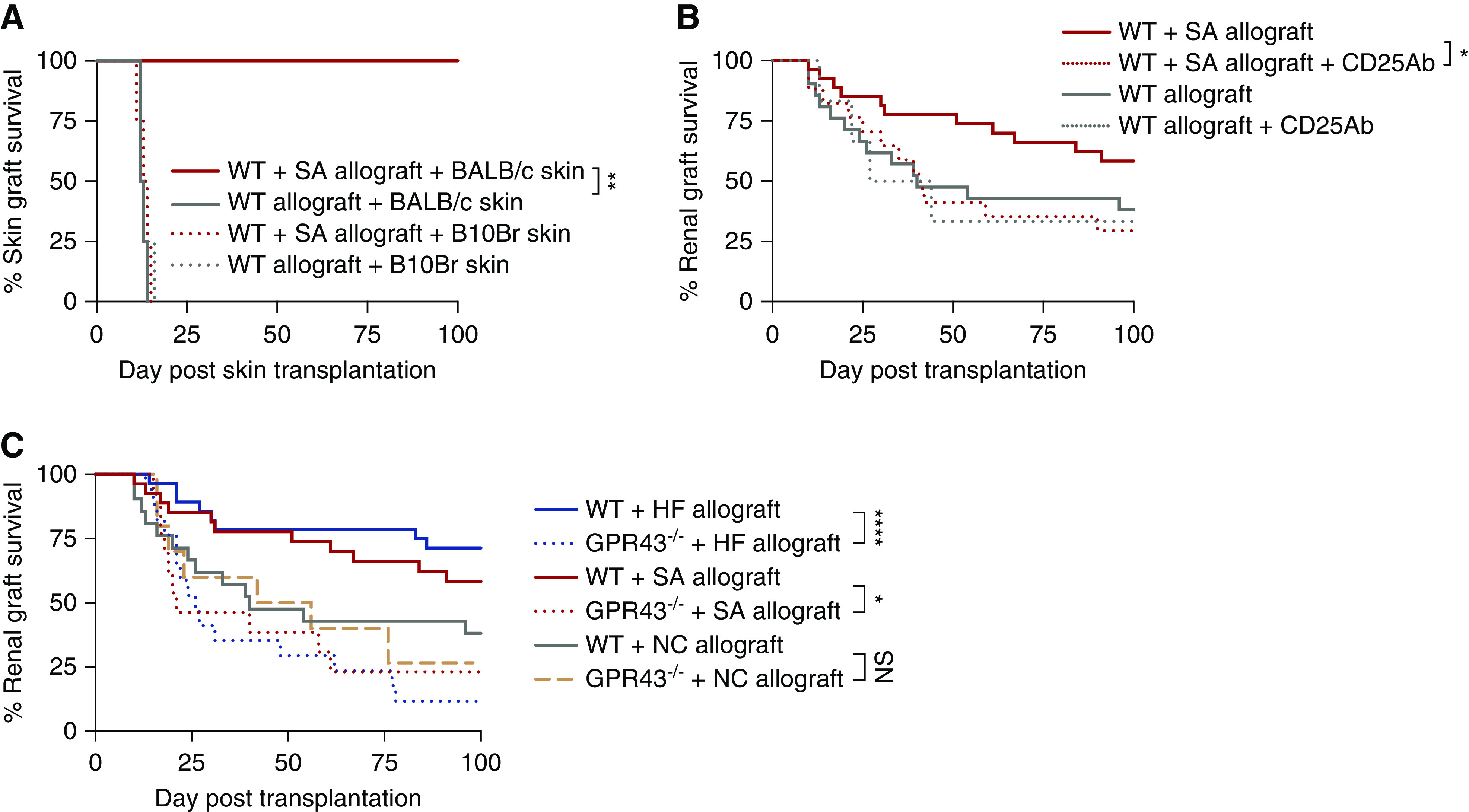
SA promoted donor antigen–specific tolerance of kidney allografts, dependent on a CD4+CD25+Foxp3+ regulatory mechanism and signaling via GPR43. (A) Skin graft survival curves for skin challenges in WT and WT+SA kidney allograft acceptors. A subset of WT (n=4) and SA-supplemented (n=4) allograft mice who survived to over day 200 after kidney transplant received skin grafts from donor-matched (BALB/c), isografts (C57BL/6), and third-party (B10Br) mice. Skin isografts remained intact on all animals, whereas third-party skin allografts were promptly rejected by day 15 by WT and WT+SA kidney allograft acceptors. WT kidney allograft acceptors rejected donor-matched skin grafts at 12–14 days, whereas WT+SA kidney allograft acceptors accepted donor-matched skin grafts for >100 days. (B) A depleting mAb to CD25 was given to WT (n=6) and SA-supplemented (n=17) allograft mice on days −2 and 0 post-transplant. The survival benefit seen in SA-supplemented allograft recipients (n=27) was annulled after depletion of CD25+ cells (P<0.05), whereas administration of anti-CD25 Ab did not alter allograft rejection kinetics in WT allograft recipients. (C) SA supplementation and HF diet was ineffective in GPR43−/− recipients of WT allografts (n=13 for SA, n=17 for HF), with no survival advantage compared with WT recipients of kidney allograft (n=21), whereas GPR43−/− allograft recipients fed NC (n=10) had graft survival comparable to WT mice fed NC. Statistical analysis by log-rank test. *P<0.05; **P<0.01; ****P<0.0001.
