Figure 2.
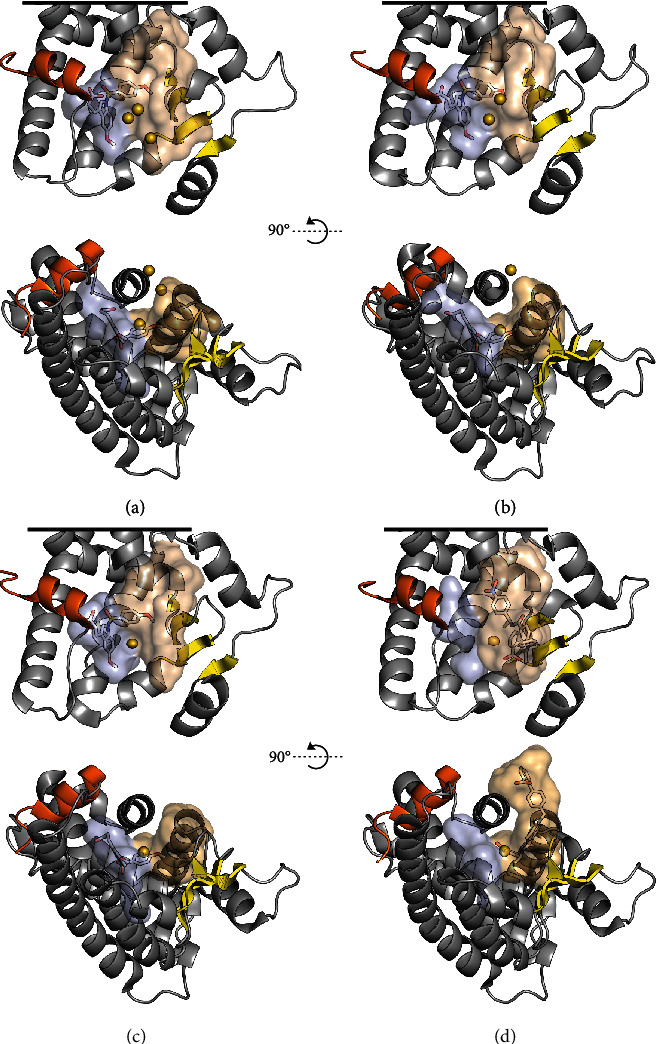
An overview of the shapes of the PPARα (a), PPARβ/δ (b), and PPARγ (c, d) LBPs and the subcavities referred to as the AF-2 pocket (light blue) and the Ω pocket (beige), lined by helix 12 (orange) and β1–β4 (yellow), respectively. For clarity, the N-terminal half of H3 and the Ω loop are hidden in the front views (top). Also, the visualizations of the LBDs have been truncated (black lines) in order to maximize the visibility of the LBP. Similarly, H2′, the Ω loop, and the N-terminal half of H3 are hidden in the top view (bottom). The sulfur atoms of the centrally located cysteines are shown as gold spheres, at 50% of their van der Waals radii. (a–c) PPARα, PPARβ/δ, and PPARγ in their respective complexes with indeglitazar (Figure S1) predominantly bound to the AF-2 pocket (PDB ID: 3ET1, 3ET2, and 3ET3, respectively) [100]. (d) PPARγ in complex with SR1664 (Figure S1) bound to the Ω pocket (PDB ID: 5DWL) [101]. The LBP surfaces were mapped with a 1.4 Å probe using HOLLOW [102], and the resulting population of probes was truncated at the solvent interface of the Ω pocket. The structures and surfaces were visualized in PyMOL (ver. 1.8.4.0) [13, 14].
