Figure 5.
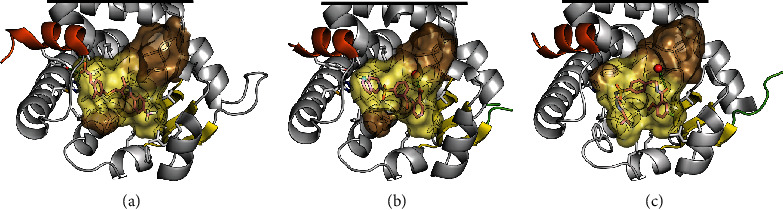
The LBP of PPARβ/δ in complex with ligands (shown with magenta carbons). To illustrate ligand-pocket interactions, the inner surface of the binding pocket is shown in brown with surface areas ≤ 3.7 Å from the ligand highlighted in pale yellow. Residues ≤ 5.0 Å from the ligand are shown with grey carbons. Plausible hydrogen bonds are shown with green dashes. H12 is shown in orange, and β1-β4 are shown in yellow. The unresolved termini of the H2–β1 loop are shown in light green. For clarity, the Ω loop and the N-terminal half of H3 (residues 224–257) are hidden. Also, the visualizations of the LBD have been truncated (black lines) in order to maximize the visibility of the LBP. (a) Classical agonist, GW0742 (PDB ID: 3TKM) [245]. (b) Partial agonist GW9371 (PDB ID: 3DY6) [242]. The side chain oxygen of Thr252 is shown as a red sphere at 50% of its van der Waals radius. (c) Compound 6 (PDB ID: 2XYX) [243]. The LBP surfaces were mapped with a 1.4 Å probe using HOLLOW [102], and the resulting population of probes was truncated at the solvent interface of the Ω pocket. The structures and surfaces were visualized in PyMOL (ver. 1.8.4.0) [13, 14].
