Abstract
The incidence of metastasis to the brain is apparently rising in cancer patients and threatens to limit the gains that have been made by new systemic treatments. The brain is considered a ‘sanctuary site’ as the blood–tumour barrier limits the ability of drugs to enter and kill tumour cells. Translational research examining metastasis to the brain needs to be multi-disciplinary, marrying advanced chemistry, blood–brain barrier pharmacokinetics, neurocognitive testing and radiation biology with metastasis biology, to develop and implement new clinical trial designs. Advances in the chemoprevention of brain metastases, the validation of tumour radiation sensitizers and the amelioration of cognitive deficits caused by whole-brain radiation therapy are discussed.
ClinicalTrials.gov:
Brain metastases — parenchymal metastases and leptomeningeal metastases (BOX 1) — most commonly arise from cancers of the lung, breast and skin (melanoma), but also occur at a reduced frequency in patients with diverse cancer types. The incidence of brain metastases is highest in patients with lung tumours. Approximately 10–25% of patients with lung cancer have brain metastases at diagnosis and another 40–50% develop them during the course of their disease, with an even greater incidence at autopsy1. Brain metastases conferred an inferior overall survival to patients with non-small-cell lung cancer (NSCLC), particularly to those who had a limited number of systemic (liver, bone and other organs) metastases2.
At a glance
Brain metastases are most common in patients with lung cancers, breast cancers or melanoma.
Treatment includes surgery and radiation therapy. Whole-brain radiation therapy (WBRT) has been shown to prevent lung cancer brain metastases, but causes cognitive decline.
In animal models of brain metastasis, tumour cells crawl outside the blood vessels and interact with an inflamed neural microenvironment to colonize the brain.
Alterations in the expression of several genes, including ERBB2, ST6GALNAC5, TCF, transforming growth factor-β (TGFB), vascular endothelial growth factor (VEGF), Serpine1 and Timp1, have modulated brain metastasis.
Chemotherapeutic efficacy for brain metastases remains disappointing.
In experimental models, brain metastases opened the blood–brain barrier (BBB) several-fold over the normal brain, but only 10% of lesions exhibited sufficient drug permeability to mount an apoptotic response to chemotherapy.
BBB-permeable drugs are needed to improve chemotherapeutic efficacy.
Prevention of brain metastasis formation in mice has been observed in response to lapatinib, vorinostat, pazopanib, signal transducer and activator of transcription 3 (STAT3) inhibitors and VEGF receptor (VEGFR) inhibitors.
New trial designs could test drugs for the prevention of brain metastases. Secondary prevention trials would determine the time to the development of a new brain metastasis in patients with either one or several existing lesions.
Radiosensitizers may improve the efficacy of radiation therapy while sparing normaltissue.
Inhibition of the neuroinflammatory response is hypothesized to protect the brain from WBRT-induced cognitive decline.
Box 1|. Leptomeningeal metastases
Also known as carcinomatous meningitis, leptomeningeal metastases develop in the microenvionment containing the cerebrospinal fluid (CSF) and the linings of the brain. This environment is not static; with metastasis it may be altered by immune cell infiltration, increased protein concentrations and reduced glucose concentrations130. When cultured with leptomeningeal tissues, metastatic melanoma and lung cancer cells invade into and degrade the leptomeninges, in contrast to glioma cells that sit on top of the tissue131. Thus, leptomeningeal metastases are distinct from primary brain tumours. In patients, spread to the leptomeninges can be accomplished by several routes, including the blood, direct extension from the brain, the venous plexus and nerves, perineural and perivascular lymphatics, and the choroid plexus. Clinically, leptomeningeal metastases confer a dismal prognosis. Leptomeningeal metastases simultaneously occur with parenchymal brain metastases in more than 50% of patients with melanoma or lung cancer. They can develop from primary lung cancer over a median of 1 year, but require more than 3 years to develop in patients with breast cancer and melanoma132. Haematological malignancies also develop leptomeningeal metastases133. Intrathecal (delivered to the CSF) chemotherapy produced responses in patients with leptomeningeal metastasis, but patient survival remained poor130. Several of the experimental brain metastasis model systems produce leptomeningeal lesions, offering hope that new pathways and therapeutics will be discovered.
Box 2|. Radiation therapy for brain metastases
Whole-brain radiation therapy (WBRT) consists of a series of treatments (fractions) of low-dose (2–3 Gy) radiation delivered to the entire brain, for patients with either one or multiple metastases. WBRT was initially validated in trials that demonstrated an improvement in patient survival from 1–2 months with supportive care versus 4–6 months when treated with radiotherapy134,135. WBRT has been tested in several clinical scenarios. No significant difference in overall patient survival was observed between WBRT versus WBRT plus stereotactic radiosurgery (SRS)136. Addition of WBRT after surgery decreased relapses at the surgical site137.
WBRT also has a role in preventing brain metastases (prophylactic cranial irradiation (PCI)). In patients with small-cell lung cancer (SCLC), PCI reduced brain metastases by 73%, increased survival from 5.4 to 6.7 months, and caused no decrease in cognitive function or emotional behaviour138. A review of randomized trials in patients with non-small-cell lung cancer (NSCLC) showed a reduction in the incidence of brain metastases, without any survival benefit139.
SRS is an alternative to surgery in which multiple convergent beams of high energy X-rays, γ-rays or protons are delivered to a discrete mass. Thus, SRS irradiates a brain metastasis but does not treat the remaining brain. SRS can be used to treat single or multiple lesions, including deep-seated surgically inaccessible lesions140,141. In retrospective analyses, SRS has an equivalent outcome to surgery140,141.
For cancers of the breast, brain metastases occur after the diagnosis of systemic metastases. In patients with metastatic disease whose tumours fall into two categories — tumours with amplification of receptor tyrosine kinase ERBB2 (ERBB2+; also known as HER2+) or triple-negative (oestrogen receptor (ER) and progesterone receptor (PR)-negative and normal levels of expression of ERBB2) tumours — the incidence of brain metastases can exceed one-third of patients. The incidence of brain metastases is lower in patients with ER-positive (ER+) metastatic tumours3–5. Worryingly, brain metastases are increasingly a first site of progression after treatment for metastatic disease in patients with ERBB2+ breast cancer, and this threatens to limit the survival gains made in systemic therapy6. For patients with triple-negative metastatic breast cancer, brain and systemic metastases often occur simultaneously7. Autopsy and imaging studies indicate that an additional 15–30% of patients with metastatic breast cancer also have brain metastases that were not diagnosed8,9.
For patients with melanoma, 50–75% have brain metastases at autopsy and two-thirds of these patients will have had symptoms and been diagnosed with brain metastases before death10. The prognosis of patients with melanoma who have brain metastases is poor, with a median survival of 2.8–4.0 months after diagnosis. Approximately 20–55% of patients with malignant melanoma die as a result of their brain metastases10.
Brain metastases are often indicated by symptoms, such as seizures, loss of motor and sensory function, cranial neuropathies and cognitive decline, and are confirmed by imaging — lesions of several millimetres in size are routinely radiographically detectable. Brain metastases are expected to become more prevalent and to clinically manifest in other cancer types as systemic therapy improves, resulting in longer patient survival and the control of metastases in other organs.
Current treatments for brain metastases are palliative and centre on surgery and radiation therapy. Surgery is a viable option for patients with only one lesion or a small number of lesions located in accessible regions of the brain and often provides rapid relief of symptoms. Two types of radiation therapy (BOX 2) are commonly used for patients: stereotactic radiosurgery (SRS) or whole-brain radiotherapy (WBRT). Both the presence of brain metastases and their treatments cause physical and cognitive morbidities, and improvements in patient survival are still measured in weeks or months. With this in mind, this Review discusses our current understanding of the biology of established brain metastases and whether recent advances in understanding the colonization of the brain by metastatic cells will enable the development of drugs that can limit the development of brain metastases. In addition, we consider the need to evaluate new drugs on the basis of whether they can treat established brain metastases or prevent them from occurring or recurring. Finally, we consider ways of improving the current standard of care (WBRT or SRS) for patients with brain metastases.
How do tumour cells colonize the brain?
The colonization of the brain by metastatic cancer cells starts with a tumour cell extravasating into the brain and eventually leads to a detectable clinical metastasis (FIG. 1). This process has been deciphered using model systems. In general, tumour cell lines have been injected into the general circulation (intracardiac or intravenous injection) or directly upstream of the brain (intra-carotid injection). The resulting brain metastases are then harvested, expanded in culture and subjected to multiple rounds of re-injection and harvesting. Using this approach, tumour cells with a tropism for growth in the brain have been derived. Experimental models of brain metastasis have been reported for lung11–13 and breast14–20 cancers, melanoma13,21–24 and other cancer types25–27. Spontaneous mouse models of brain metastasis that emanate from a primary tumour have been less frequently reported20,28. The relevance of certain models to aspects of the development of human brain metastases, such as rates of proliferation or apoptosis, the neuroinflammatory response29 and drug resistance11, have been reported. These models probably represent examples of the heterogeneity of human brain metastatic progression; additional models covering poorly understood facets of brain metastasis, such as chemotherapeutic resistance, cognitive dysfunction and radiation resistance, are still needed.
Figure 1|. Steps in the development of brain metastases in an animal model.
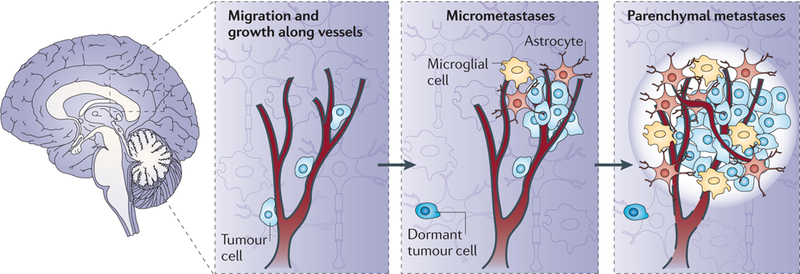
Brain metastatic cancer cells traverse the vascular system and use the outside of vessels as a site of adhesion and migration13,20. Later, the tumour cells use the inflamed brain microenvironment as a niche. Tumour cells interact with activated microglia (macrophage-like cells, shown in yellow) and astrocytes (shown in orange), which provide support for neuronal function. As the metastasis expands, neuronal damage ensues. The brain microenvironment also contains damaged axons, oedema (white halo) and vascular changes (such as the disruption of the blood-brain barrier, indicated by dashed black lines). Both vessel co-option and angiogenesis have been reported in brain metastasis. Dormant solitary tumour cells can also reside in the brain39, constituting a potential source for the development of additional metastases.
Interactions between tumour cells and the brain micro-environment.
In 1889 Paget described metastasis as an interaction between a tumour cell (the ‘seed’) and a congenial microenvironment (the ‘soil’)30. At least three microenvironments have been implicated in brain metastatic colonization: the perivascular niche, the brain parenchyma and the cerebrospinal fluid (CSF) or the leptomeningeal niche (BOX 1). Early after injection, breast and melanoma brain-tropic cell lines intimately associate with the outside surface of a blood vessel. Tumour cells elongate their shape along the vessels, adhere to the vascular basement membrane via β1 integrins, and proliferate and invade while on top of the vascular basement membrane20. Similar results were reported for a brain-tropic Lewis lung carcinoma line early after carotid injection, which was followed by a brain parenchymal growth pattern12.
The second metastatic niche, the brain parenchyma, is altered by neuroinflammation29,31. Histological analysis of resected human brain metastases revealed tumour cells interdigitated with activated microglia and astrocytes29,32,33. These data indicate that metastases might form from the convergence of small micro-metastases that are encased in the brain parenchyma. Indeed, activation of astrocytes and microglia is widely evident around experimental brain metastases29,33,34. Both in vitro and ex vivo studies support a functional interaction of cancer cells and the neural microenvironment. For example, when tumour cells embedded in matrix were cultured next to a brain slice, microglia accumulated at the point of contact, associated with the tumour cells and facilitated their invasion into the slice35. Astrocytes can enable the growth of brain-tropic tumour cell lines in co-culture experiments29,33. Seike et al.33 have proposed a ‘vicious cycle’ in which tumour cell factors, such as macrophage inhibitory factor, interleukin-8 (IL-8) and plasminogen activator inhibitor 1, activate astrocytes that, in turn, produce proliferative factors for the tumour cells, including IL-6, IL-1β and tumour necrosis factor33.
Complex vascular changes are evident during parenchymal colonization. Although the brain has a rich supply of blood vessels, vessel density is lower in experimental metastases than in normal brain, but vessels are dilated and tortuous in the metastases15,20. It also seems that metastasis-specific patterns exist, as human melanoma and lung brain metastases have a lower vessel density than brain metastases from breast cancers36. Co-option of the existing vasculature has been reported20,21, and the role of neo-angiogenesis during colonization of the parenchyma has been debated25,37,38. The role of anti-angiogenic therapy, through the inhibition of the vascular endothelial growth factor (VEGF) receptor (VEGFR) has been reported in preclinical models and the results have been mixed. Using the Mel57-VEGF-A melanoma cell line, brain metastases became undetectable by magnetic resonance imaging (MRI) owing to permeability changes, but small non-angiogenic lesions persisted, showing evidence of vessel co-option21. In a prostatic cancer model, brain metastases demonstrated a reduced central vascular bed but retained a rim of increased blood volume25. These findings probably reflect the fact that the functions of VEGF and angiogenesis seem to be complex in brain metastasis. For example, the overexpression of a splice variant of VEGFA, VEGF-A165, in a melanoma cell line accelerated the invasive growth of brain metastases37. Central necrosis, dilation of blood vessels and vascular permeability were also evident, but sprouting angiogenesis was absent.
Non-progressive colonization: dormancy.
Dormant tumour cells have been described in the brain. Using double-contrast MRI (DC-MRI) of 231-BR breast cancer cells expressing enhanced green fluorescent protein (EGFP) and loaded with micron-sized iron oxide particles, the fate of single metastatic cells was serially imaged in the mouse brain. Proliferation of the tumour cells divides the iron oxide particles between daughter cells, resulting in an undetectable concentration and enabling the detection of the fluorescent EGFP lesion. For every overt fluorescent green brain metastasis formed, three cells remained dormant39, providing a considerable pool of tumour cells to potentially awaken and lead to further relapses.
Molecular pathways mediating brain metastasis.
The best evidence for gene expression changes during metastasis to the brain comes from a comparison of tissue blocks containing the primary tumour with a surgically resected brain metastasis from the same patient. Using these rare resources, differences were reported in the expression of stem cell markers40, receptor tyrosine kinases40–43, hormone receptors44, cyclooxygenase 2 (REF 43), proteins involved in apoptosis43 and DNA repair enzymes45,46. The methylation of genes such as secretoglobulin family member 3A, member 1 (SCGB3A1; also known as HIN1) and retinoic acid receptor-β (RARB) was increased in metastases from the brain, as well as lung and bone47. In addition, DNA sequencing of a matched primary tumour and brain metastasis from a patient with basal-like breast cancer indicated that the metastasis and the tumour shared many mutations and that the metastasis probably developed from a few cells in the primary tumour; brain metastasis-specific DNA copy number alterations and mutations were also identified48. Among unmatched samples of primary tumours and brain metastases, reduced expression of the NM23, KISS1, KAI1, BRMS1 and MKK4 metastasis suppressor genes49, the BCL2 anti-apoptotic gene50 and the Notch-target transcription factor HES1 (REF 51) was reported. Conversely, high expression of hexokinase 2 (HK2)52 and phosphorylated signal transducer and activator of transcription 3 (STAT3)53 were seen in brain metastases. All of these trends represent potential leads for the functional modulation of brain metastatic potential. To reveal additional pathways that are involved in metastasis to the brain, gene expression changes between experimental brain-tropic and parental tumour cell lines have been identified. Only a few pathways have been functionally confirmed in brain metastasis assays to date using gene overexpression or underexpression in brain-tropic cell lines. Many of these genes have previously been implicated in metastasis to other organs, suggesting that brain colonization results from both general and site-specific metastatic pathways.
Overexpression of ERBB2 in the 231-BR breast cancer cell line had no effect on the number of micro-metastases per brain section, but increased the number of large metastases (comparable to a 5 mm lesion in a single dimension in a human brain) by 2.5–3-fold54. Thus, ERBB2 overexpression had no effect on the initial stages of tumour cell arrival or growth, but promoted the final steps of metastatic colonization in the brain. In lung cancer, overexpression of the receptor tyrosine kinase MET and its ligand hepatocyte growth factor (HGF) in NCI-H460 tumour cells promoted widespread metastasis, including to the brain55.
In lung cancer, activation of the WNT pathway has been linked to bone and brain metastasis. Binding of WNT ligands to their receptor stabilizes β-catenin (encoded by CTNNB1), which binds to the transcription factors of the lymphoid enhancer-binding factor (LEF) transcription factor (TCF) family. A TCF-related gene signature predicted lung cancer metastasis-free survival but not breast cancer metastasis-free survival. Expression of dominant-negative TCFs inhibited the brain and systemic metastasis of lung cancer cell lines, and was mediated by alterations in LEF1 and homeobox protein HOXB9 (REF 56).
A potential site-specific brain metastatic pathway involves an α−2,6-sialyltransferase ST6GALNAC5 (also known as α-N-acetylgalactosaminide). ST6GALNAC5 was identified by its overexpression in brain, but not in bone- or lung-tropic breast cancer cell lines — lectin staining for ST6GALNAC5 was observed in 50% of brain metastases compared with 18% of lung metastases. Sialyltransferases are thought to affect cell–cell interactions through the sialylation of gangliosides and glycoproteins. Knock down of ST6GALNAC5 reduced tumour cell line migration across artificial blood-brain barriers (BBBs) in vitro and brain metastasis in animal models17.
Cytokines and their signalling pathways participate in metastatic colonization in the brain. Transforming growth factor-β (TGFβ) is a cytokine that has been widely reported to inhibit the initiation of tumori-genesis but to also stimulate tumour progression and metastasis. Murine B16 melanoma cells produced exclusively leptomeningeal metastases; overexpression of TGFβ2 induced parenchymal micrometastases but had no effect on the leptomeningeal lesions23. The STAT signalling pathway, which is downstream of many cytokines, was activated in brain metastases. Transfection of STAT3 into A375 brain-tropic melanoma cells increased the incidence of brain metastases, as well as their blood vessel density, and decreased the survival of the injected animals53. STAT3 promotion of melanoma brain metastasis is linked to decreased expression of the suppressor of cytokine signalling 1 (SOCS1), which is a negative regulator of cytokine signal transduction24.
Potential microenvironmental contributions to brain metastasis include the expression of proteases within the parenchyma and by the invading tumour cells. Transgenic overexpression of plasminogen activator inhibitor 1 (Serpine1) and tissue inhibitor of metalloproteinase 1 (Timpl) in mouse brains reduced the incidence of brain metastasis26,27. Similarly, microRNA-1258 inhibited tumour cell heparanase expression and decreased experimental brain metastasis57.
Future investigations will no doubt identify other pathways that are essential for the colonization of the brain by metastatic cells.
Why chemotherapy usually fails
Poor chemotherapeutic permeability and efficacy.
The clinical data on the responsiveness of brain metastases to standard chemotherapy and molecularly targeted drugs are unambiguously disappointing, with only a handful of clinical responses to most standard cytotoxic drugs58–64. Some clinical responses to temozolomide have been reported in patients with melanoma brain metastases65. Capecitabine (Xeloda; Roche), a nucleotide-based chemotherapeutic, has produced responses alone and in combination with other drugs in patients with breast cancer brain metastases66,67. Disappointing results were also reported when chemotherapy was added to WBRT68.
Epidermal growth factor receptor (EGFR) inhibitors produced clinical responses in 10–30% of patients with brain metastases from NSCLC69,70. However, the concentration of erlotinib (Tarceva; Genentech) in the CSF was 6% of plasma levels71. Concerns have also been raised about a high rate of brain metastases following a systemic response to EGFR inhibitors. For example, 43% of patients with a partial response to gefitinib (Iressa; AstraZeneca) developed brain metastases after a mean follow-up of 27 months72.
For patients with ERBB2+ breast cancer, the humanized monoclonal antibody trastuzumab (Herceptin; Genentech) is the standard of care combined with chemotherapy. Considerable clinical data have accumulated on the incidence of brain metastases and the outcome of these patients. In one study, 50% of patients with breast cancer who had systemic metastatic ERBB2+ disease were responding to chemotherapy or had stable systemic disease when brain metastases were diagnosed, and 50% of patients died of progressive brain metastases6. A meta analysis of trials of trastuzumab in the adjuvant setting showed an increased relative risk of brain metastasis of 1.57 (REF. 73), indicating that treatment with this drug ahead of the diagnosis of distant metastatic disease seems unlikely to be able to prevent the development of brain metastases. Trastuzumab efficacy in the brain is probably diminished by poor penetration. The ratio of trastuzumab levels in the CSF and serum was 1/420 when tested at baseline, and rose to 1/49–1/76 post-radiation treatment: these levels are still considered sanctuary site levels74. Lapatinib (Tykerb; GlaxoSmithKline) was approved in combination with capecitabine in patients with ERBB2+ metastatic breast cancer who have progressed on trastuzumab and chemotherapy. Lapatinib shows restricted but improved brain uptake compared with that of trastuzumab, reaching levels of up to one-quarter of those in plasma75. In a Phase II trial, the shrinkage of ERBB2+ brain metastases was minimal with lapatinib or lapatinib and capecitabine (6% and 20% partial response rates, respectively), with additional patients experiencing stable disease64. The fact that brain metastases are less frequent in patients with ER+ breast cancer might reflect the fact that tamoxifen, a selective ER modifier, can cross the BBB76.
A recombinant humanized monoclonal antibody against VEGF, bevacizumab (Avastin; Genentech/Roche), was administered to patients with NSCLC brain metastases. Among 106 evaluable patients, two Grade 5 pulmonary haemorrhages were reported, 24.5% of participants discontinued the study owing to an adverse event and 34.9% discontinued owing to disease progression77. Inhibitors of VEGFR (and other receptor tyrosine kinases) have been tested in patients with brain metastases from renal cancer. In the US Food and Drug Administration expanded access programme, 4% of the patients treated with sorafenib (Nexavar; Bayer) showed a clinical response in the brain78.
The blood-tumour barrier (BTB).
Although metastatic disease is generally considered incurable, responses in the brain seem to be even lower than those at systemic sites. At least two theories may explain the disappointing chemotherapy clinical data. First, metastatic tumour cells in the brain are more resistant to chemotherapy than systemic metastases. Resistance may result from their late development after multiple rounds of prior chemotherapies, and could reflect accumulated mutations. Second, the remnants of the BBB prohibit adequate amounts of chemotherapy from reaching the metastases. The BBB consists of the brain vasculature and the surrounding architecture, which severely limits the access of many molecules to the brain (FIG. 2a). The endothelial cells of the BBB express a plethora of active transporters. Together, these transporters act as efflux pumps to send substances out of endothelial cells and back into the circulation, away from the brain parenchyma. Under normal conditions, the molecules that most readily pass from blood into the brain are small and lipophillic and are not recognized by the active efflux pumps79. These compounds diffuse across the multiple cell membranes of the BBB into the brain parenchyma. Other necessary substances, such as glucose, amino acids, vitamins, nucleic acid precursors and some hormones, are moved into the brain by facilitated diffusion80. Most standard chemotherapeutics have been shown to be substrates of one or more of the active efflux transporters81,82 (TABLE 1).
Figure 2|. The blood–brain barrier (BBB) and its role in drug uptake.
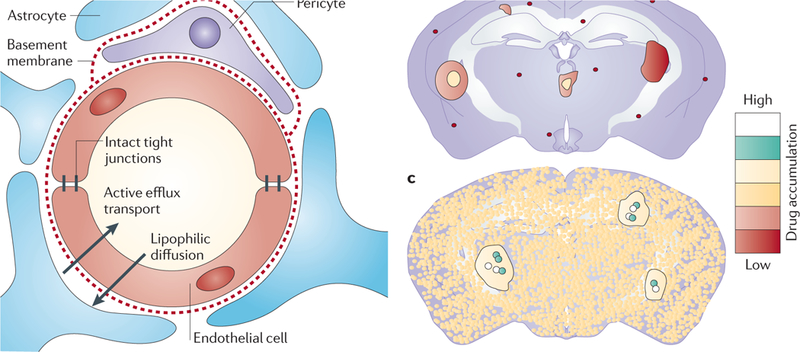
a | The BBB protects the normal brain by permitting access to only select substances. Endothelial cells are surrounded by pericytes, a basement membrane and the feet of astrocytes, all of which function as a barrier. Endothelial cells in the normal brain are tightly connected by continuous tight junctions and express multiple efflux pumps to push unwanted substances back into the bloodstream107. b | The results in mice harbouring brain metastases that were given an intravenous injection of radiolabelled drug (paclitaxel or doxorubicin) are illustrated. Drug uptake into normal brain and brain metastases was quantified by autoradiography of tissue sections. Although most brain metastases accumulated a higher concentration of the drug than cells in the normal brain, heterogeneous levels of drug uptake were observed and the highest concentration was only observed in ~10% of the lesions15. c | Vorinostat, a histone deacetylase inhibitor, was administered to mice with brain metastases (as described in part b). Drug uptake throughout the brain is evident, as well as heterogeneous increased uptake in metastases.
Table 1|.
Heterogeneity of drug efflux pumps for chemotherapeutic and molecular therapeutic agents
| Drug class | Drug | P-glycoprotein (ABCB1) |
BCRP (ABCG2) |
MRP1–7 (ABCC1–10) |
OAT | OCT and OCTN |
OATP | ENT or CNT |
Other |
|---|---|---|---|---|---|---|---|---|---|
| Vinca alkyloids | Vinblastine, vincristine and vinorelbine |
++* | − | + 7‡ | |||||
| Anthracyclines | Doxorubicin | ++ | + | + 1, 2, 6 and 7 | + OCT6 | + RALBP1 | |||
| Daunorubicin | ++ | + | + 1, 6 and 7 | + RALBP1 | |||||
| Epidophyllotoxins | Etoposide | ++ | + | + 1, 2, 3 and 6 | |||||
| Taxanes | Paclitaxel and docetaxel | ++ | - | + 2 and 7 | + 2 | + 1B3 | |||
| Tyrosine kinase Inhibitors |
Axitinib, dasatinib, lapatinib, sunitinib and tandutinib |
++ | + | ||||||
| Erlotinib | ++ | + | + 7 | + 1 and 3 | + 1B3 | ||||
| Gefitinib | ++ | + | + 1 and 3 | ||||||
| Imatinib | ++ | + | + 7 | + 1 and 3 | |||||
| Sorafenib | + | ++ | |||||||
| Camptothecins | Topotecan | ++ | + | + 4 | + 3 | ||||
| Irinotecan (SN-38) | ++ | + | + 1, 2 and 4 | + 1B1 | |||||
| Thiopurines | 6-mercaptopurine | + | + 4 and 5 | + 3 | |||||
| 6-thioguanine | + 4 and 5 | + 3 | |||||||
| Nucleic acid precursors | 5-fluorourcil | + 5 and 8 | + 3 | ||||||
| Gemcitabine | + 4 and 5 | + ENT1 and ENT2, and CNT2 |
|||||||
| Other | Melphalan | + LAT1 | |||||||
| Cisplatin | + 2, 5 and 6 | + 1 and 2 | |||||||
| Methotexate | + | + | + 1, 2, 3 and 5 | + 3 | + 1B1 |
BCRP, breast cancer resistance protein; CNT, concentrative nucleoside transporter; ENT, equilibrative nucleoside transporter; LAT1, large neutral amino acids transporter, small subunit 1; MRP1–7, multidrug resistance-associated protein 1–7; OAT, organic anion transporter 3 (also known as SLC22A8); OATP, organic anion transporting polypeptide; OCT, organic cation transporter; OCTN, organic cation/carnitine transporter; RALBP1, RAL binding protein 1.
+ to ++ indicates the degree to which a drug is subject to efflux transport. – indicates that the drug is not transported.
Numerals indicate the transporter isoform at the blood–brain barrier.
The brain metastasis research field has debated the extent to which metastasis disrupts the BBB, forming a BTB. Imaging studies showing a greater uptake of contrast agents in brain metastases compared with surrounding brain tissue have suggested that the barrier is open, whereas chemotherapeutic efficacy data suggest that, if the barrier is open, it is not open enough to permit sufficient drug accumulation. It is also not clear whether the pharmacokinetics of drug uptake into primary brain tumours are identical to those of brain metastases. Recent pharmacokinetic studies of two experimental brain metastasis models revealed that, although most metastases have some increased permeability compared with normal brain, heterogeneous uptake levels can occur (FIG. 2b) and only 10% had sufficient permeability to show a cytotoxic response to chemotherapy15. Median drug levels in experimental brain metastases remained a log lower than those achieved in systemic metastases. In agreement with these data, neither paclitaxel nor doxorubicin significantly decreased experimental brain metastasis in a mouse model of breast cancer. These data strongly support the conclusion that brain-permeable drugs are needed if chemotherapy is to have a prominent role in the prevention or treatment of brain metastases15.
Drug efflux pumps markedly contribute to the observed lack of brain permeability (TABLE 1). Using knockout mice for Abcb1 and Abcg2, uptake of axitinib, dasatinib (Sprycel; Bristol-Myers Squibb), erlotinib, gefitinib, imatinib (Glivec; Novartis), lapatinib, sorafenib, sunitinib (Sutent; Pfizer) and tandutinib in the normal brain was substantially increased, with Abcb1 having a dominant role for most agents except sorafenib. Elacridar, an inhibitor of both pumps, was almost as efficacious in increasing brain sorafenib concentration as the double transporter Abcb1;Abcg2 knockout, whereas it was less potent at increasing the concentrations of gefitinib in the brain83,84. Roles of other BBB and BTB efflux pumps remain incompletely characterized and may contribute to inadequate drug permeation of brain metastases.
The rate of uptake of a dextran marker compared with a chemotherapeutic drug in experimental brain metastases was closely correlated15, suggesting that the overall architecture of the BTB contributes to altered permeability along with drug-specific transporters. Using immunofluorescence, the vasculature of permeable experimental brain metastases was surrounded by greater numbers of pericytes, as shown by desmin staining, whereas a drug transporter protein, P-glycoprotein (ABCB1) was comparably expressed in permeable and nonpermeable lesions15. The correlation of increased pericyte coverage and permeability was unexpected, as pericytes contribute to the BBB-protective function85,86. However, under hypoxic conditions pericytes can collaborate with astrocytes to exacerbate BBB disruption87. Another role for astrocytes in chemotherapeutic permeability was also suggested by the adhesion of astrocytes to tumour cells in vitro, thus protecting them from chemotherapeutic compounds by altering gap junctions and so resulting in calcium sequestration34.
Validation of brain-permeable compounds
It is expected that continued mechanistic insight into the brain metastatic process will identify additional druggable targets. Multiple new approaches to prevent and treat brain metastases are now underway (TABLE 2). For drug-related approaches, preclinical data using brain-tropic model systems have addressed three general questions. First, can brain metastasis-permeable drugs be identified? Increasingly, the pharmaceutical industry is now considering brain permeability when choosing a lead compound. In general, brain permeability is optimal in a compound with a low molecular mass (<450 Da), moderate lipophilicity (calculated logP<5), a limited number of hydrogen bond donors (less than three) and acceptors (less than seven), neutral or basic pKa (7.5–10.5) and limited polar surface area (<60–79 Å)79. Second, do brain-permeable drugs have efficacy as a treatment for established brain metastases, as assessed in ongoing clinical trials, or in the prevention of the colonization of the brain by metastatic cells? Third, can brain-permeable drugs synergize with radiation therapy?
Table 2|.
New approaches to prevent or treat brain metastases
| BBB-permeable and effective therapeutics |
Vorinostat, lapatinib, pazopanib, JNJ-2887–1063, WP1066 and epothilones |
| Brain-tropic viruses | Vesicular stomatitus virus |
| Increase the permeability of the BBB | Angiopep 2, phosphodiesterase 5 inhibitors, radiation and BBB disruption |
| Radiation sensitization of tumour cells | Multiple kinase inhibitors, vorinostat and DNA damage response inhibitors |
| Protection of normal brain from WBRT-induced neurocognitive deficits |
Fenofibrate, pioglitazone and ACE inhibitors |
ACE, angiotensin-converting enzyme; BBB, blood–brain barrier; WBRT, whole-brain radiotherapy.
Vorinostat (Zolinza; Merck) is a histone deacetylase inhibitor and has been approved for the treatment of recurrent cutaneous T cell lymphoma. It modulates gene expression by altering histone-dependent chromatin conformation, and also affects the acetylation of other proteins. When injected into mice with breast cancer brain metastases, vorinostat crossed the normal BBB and exhibited heterogeneous twofold to three-fold greater uptake in metastases relative to normal brain (FIG. 2c). Administration of vorinostat on day 3 of a 25-day experiment reduced the formation of large metastases by 62%, and micrometastases by 28%, which is consistent with its brain permeability. The efficacy of vorinostat sequentially decreased to insignificant levels as the delay lengthened for administration; if the drug was started on day 14, when micrometastases and occasional large metastases had formed, it had no significant inhibitory effect88. These data highlight a disconnection between the prevention and the treatment of a brain metastasis (discussed below). Another issue is the advancement of a drug into trials for treating brain metastases when its clinical history in the systemic metastatic setting is mixed. Vorinostat showed disappointing clinical activity against metastatic breast cancer89, and in patients with advanced lung cancer few responses were observed using vorinostat as monotherapy. However, vorinostat synergized with carboplatinum and paclitaxel to increase response rates, with a trend towards improved progression-free survival90.
We observed an additional activity of vorinostat as an inducer of DNA damage. Vorinostat induced DNA double-strand breaks in brain-tropic breast cancer cells in vitro and in vivo, with reduced expression of the DNA repair protein RAD52 (REF. 88), suggesting a potential synergy with radiation. Mouse survival was increased using the combination of vorinostat and 5 Gy radiation following intracerebral implantation of brain-tropic breast cancer cells91. The combination of vorinostat and radiation has progressed to a Phase II trial (clinical trial number: NCT00838929; see ClinicalTrials.gov(see Further information)).
Lapatinib, an ERBB2 and EGFR kinase inhibitor, prevented the formation of metastases by brain-tropic breast cancer cells that were transfected with ERBB2 by 53% (REF 92). Like vorinostat, lapatinib administration began soon after tumour cell injection and continued throughout the experiment. Phospho-ERBB2 staining of brain metastases was significantly reduced in lapatinib-treated animals, confirming that the drug hit its target in vivo. JNJ-28871063, another ERBB2 kinase inhibitor, has been reported to accumulate in the brain at higher levels than in plasma and to improve the survival of mice with intracranially implanted tumour cells93.
A brain-permeable STAT3 inhibitor, WP1066, was tested in mice with intracerebrally inoculated melanoma metastases. The overall survival of these mice increased from 15 days to over 78 days. The drug affected the interaction of the tumour cells with the brain microenvironment, reducing tumour cell production of TGFβ, VEGF and other chemokines. It also inhibited the proliferation of regulatory T (Treg) cells and increased cytotoxic T cell responses94. The effect of the compound on the inhibition of STAT3 activation in the tumour has not been reported.
Sagipilone is a BBB-permeable epothilone with a long half-life in the brain. It inhibited the intracerebral growth of MDA-MB-435 cancer cells approximately five-fold in contrast to the nonsignificant effect of paclitaxel. Sagipilone also significantly inhibited the intracerebral growth of Lu7187/7,466 NSCLC cells compared with the nonsignificant effects of temozolomide95.
Pazopanib (Armala; GlaxoSmithKline), an inhibitor of VEGFR1–3, α-type platelet-derived growth factor (PDGFRA), PDGFRB and KIT, has anti-angiogenic activity and is approved for the treatment of renal cancer. Recent experiments indicate that pazopanib also inhibits the serine/threonine protein kinase activity of BRAF, particularly wild-type BRAF that is activated by ERBB2 overexpression16. Pazopanib prevented the development of brain metastases in ERBB2-transfected 231-BR breast cancer cells by 73% and the size of brain-tropic ERBB2-transfected MCF-7 breast cancer brain metastases by twofold16. Interestingly, immunohistochemistry indicated that the phosphorylation levels of ERK and MEK were reduced in the pazopanib-treated brain metastases, suggesting that the inhibition of BRAF signalling was a contributing factor, but vascular density was unchanged.
Could brain-tropic viruses have a role in the treatment of brain metastases? Vesicular stomatitus virus attacked an intracranially implanted mouse mammary tumour, as well as a primary glioma, with the port of entry being a disrupted BTB96. Reovirus type 3 is a naturally occurring replication-competent virus that usurps the RAS signalling pathway of tumour cells with cytotoxic effects. In vivo, reovirus inoculation into intra- cerebrally implanted breast tumour metastases reduced their size and extended survival. Side effects included a mild local inflammation and mild hydrocephalus97.
Successful chemotherapy for brain metastasis
Most clinical trials for brain metastases enrol patients with diagnosed brain lesions and either test an experimental therapeutic in patients who have progressed after WBRT treatment, or test the therapeutic in combination with WBRT. Trial end points include shrinkage or stabilization of the metastases and compatibility with systemic therapy. Little effect on patient survival has been achieved. Measurements of cognition are only infrequently attempted and even fewer trials use a comprehensive battery of tests to establish cognitive side effects. Often, patients with brain metastasis are enrolled into a single trial and are not enrolled on the basis of tumour type, despite the fact that clinical and molecular features separate these diseases. The emerging pharmacokinetic data suggest two avenues for future chemotherapeutic development: first, the identification of BBB-permeable drugs with preventive or cytotoxic activity; and second, methods to increase BBB permeability to permit brain penetration of less permeable but effective therapeutics.
Is the prevention of brain metastases a better drug target?
Although arguments rage that preclinical models fail to predict clinical trial results, the data for brain metastases are currently compatible. Simply, the shrinkage of established lesions with standard cytotoxic drugs or molecularly targeted drugs has not been achieved pre-clinically, or clinically in most cases. The lack of a therapeutic benefit makes intuitive sense when considering the at-best partial brain permeability of most drugs, the partial permeability of the BTB, the fact that many molecular therapeutics are cytostatic not cytotoxic, the number of tumour cells in a several-millimetre lesion that must be killed to achieve a clinical response and the increased hydrostatic interstitial fluid pressure from oedema that can limit drug uptake. The most profound preclinical observation that has been reported, however, is that prevention of the outgrowth of brain metastases is partially achievable. In a prevention scenario, a brain- permeable drug could potentially reach and control the outgrowth of a more limited number of micrometastatic tumour cells. This hypothesis is supported by the time course data for vorinostat (as discussed above)88. Almost all of the preclinical compounds that have been reported to date were tested in a prevention setting. Limited clinical trial data also support the hypothesis that prevention of brain metastases is more achievable than shrinkage of an established lesion — a retrospective analysis of the clinical trial data from sorafenib in patients with renal cancer brain metastases78 revealed a 75% prevention of brain metastasis development98 (compared with a 4% clinical response rate on established metastases78, as discussed above). Lapatinib exhibited low response rates in trials enrolling patients with breast cancer who had established brain metastases that expressed ERBB2. However, although direct comparisons cannot be made, long-term follow-up from the metastatic breast cancer (MBC) trial of lapatinib plus capecitabine versus capecitabine alone indicated a significant reduction in the brain as the first site of relapse99, which is a preventive effect. A retrospective review of patients with advanced NSCLC who were initially treated with gefitinib showed a 25% incidence of development of brain metastases over a median of 42 months, which is considered low by historical estimates and is superior to traditional response rates for established lesions100.
For many primary prevention trials, brain metastases are quantified only when they are the first site of recurrence and later brain events are ignored, allowing conclusions to be drawn on the basis of partial data. Moreover, such trials are expensive and require years of patient follow-up. This underlines the need to identify patients at the highest risk of developing brain metastases for enrolment. Several methods for identifying patients who are most likely to develop brain metastases have been reported, including a WNT gene pathway signature56 and a three-protein immunohistochemical signal101 in lung cancer and a clinical nomogram for breast cancer102, but none is in common use.
Secondary prevention trials represent an as yet untried method for examining the efficacy of drugs at preventing brain metastases. This trial design would enrol patients who have been diagnosed with and treated (excluding WBRT) for brain metastases who are therefore at a high risk of developing further brain metastases. Patients would receive systemic therapy and would be randomized to placebo or an investigational agent. The relevant end point would be time to the development of a new brain metastasis rather than shrinkage of the existing lesion. Other end points would include compatibility with systemic treatment, patient survival and cognitive function. A graded prognostic assessment for patients with breast cancer brain metastases separated patients into groups with survival ranging from 4.2 months to 32.3 months103. The 32.3-month group, defined on the basis of performance status, ERBB2 and hormone receptor expression, and number of brain metastases, could enable the selection of longer term survivors who would be ideal candidates for secondary prevention trials. It remains to be determined whether drugs passing through the BBB will cause greater cognitive losses.
Bypassing the BBB.
Several interesting preclinical leads have emerged that can push non-brain-permeable drugs past the BBB. Some use the existing structure of the BBB, such as angiopep 2, a 19-amino acid peptide that binds the low-density lipoprotein receptor-related protein (LRP) receptors at the BBB, resulting in facilitated transport across the BBB104. Initial work with angiopep 2-conjugated paclitaxel demonstrated >50-fold enhanced delivery across the BBB104, and this agent has entered clinical trials. The role of pathological signalling in the BBB compartment is also under investigation. Phosphodiesterase 5 inhibitors, such as vardenafil (Levitra; Bayer), alter the endocytic pathway of endothelial cells. In vivo, vardenafil increased trastuzumab uptake in intracranially implanted ERBB2+ breast tumour cells by twofold105. Radiation is the best studied BBB permeabilizer, although it has not yet been studied in a brain metastasis model106. Multiple pathways may mediate the radiation permeabilization of the BBB, including endothelial cell loss, reduced P-glycoprotein expression, VEGF production by activated astrocytes and binding of leukocytes to the damaged endothelia. Finally, BBB disruption is achieved by intracarotid infusion of a hyperosmotic agent to reversibly shrink brain endothelial cells and open their tight junctions; this strategy has been used most successfully for primary central nervous system lymphoma107.
Improving radiotherapy for brain metastases
Radiation therapy is the most commonly used procedure for the treatment of brain metastases. Overall goals for future research include optimizing the efficacy of radiation therapy against metastatic tumour cells compared with normal brain cells, and preventing the cognitive losses that a proportion of patients suffer.
Radiosensitizers.
Radiosensitizers are chemicals or biological agents that increase the lethal effects of radiation on the tumour without causing additional damage to normal tissue. Multiple drugs have been tested for radiation sensitization, including pyrimidine analogues, hypoxic cell sensitizers, traditional chemotherapeutic agents, kinase inhibitors and anti-angiogenic agents108,109. Overall, these studies have produced mixed results — some have shown a slight survival benefit but most have not shown a difference in survival — and have not been strong enough to bring any of these agents into routine clinical care. Multiple molecular therapeutics have been preclinically tested in vitro and in vivo on a variety of cancer cell types for the sensitization of radiation with promising results, including inhibitors of MAPK, poly(ADP ribose) polymerase (PARP), and serine/threonine protein kinases PLK1, CHK1 and CHK2 (REFS 110–113) (FIG. 3). Although it is hoped that these newer molecular therapeutics may be a long-awaited clinical advance in radiosensitization, it is also important to question why promising preclinical data on radiosensitizers have so far failed to translate to the clinic. One possible clue emanates from a gene expression analysis of a glioblastoma cell line grown in vitro, as a subcutaneous tumour or intracranially. Gene expression after radiation therapy was dramatically different between these situations, confirming the importance of the appropriate microenvironment114. Testing of potential radiosensitizers in more relevant brain metastatic models is needed.
Figure 3|. Pathways mediating radiation sensitization.
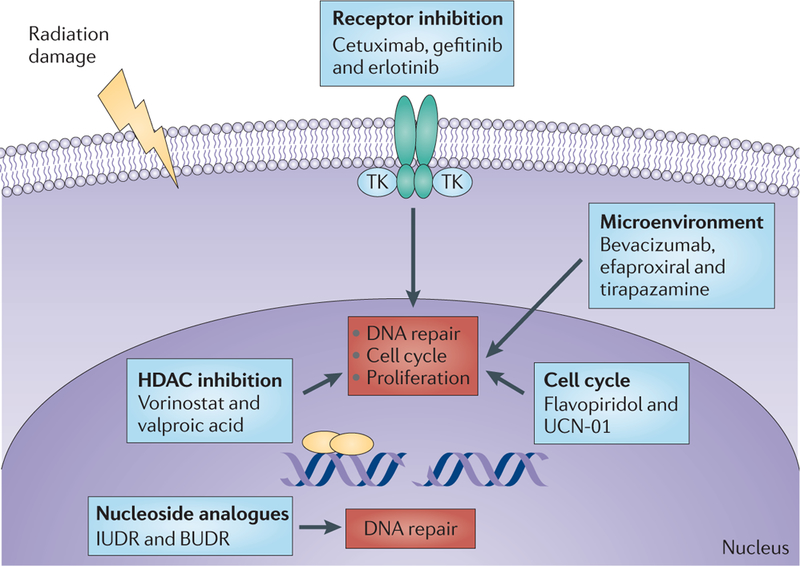
Within irradiated turn our cells the most lethal DNA damage is that which results in DNA double-strand breaks (DNA DSBs). Unrepaired DNA DSBs lead to cell cycle arrest, which if prolonged can lead to cell death. Numerous putative radiation sensitizers affect multiple aspects of this cascade, including DNA repair enzymes, cell cycle checkpoints and cellular proliferation. The inhibition of targets within the tumour stroma can also sensitize tumour cells to radiation142. For example, the inhibition of growth factor receptors on blood vessels can increase radiation sensitivity in tumour cells. BUDR, bromodeoxyuridine; HDAC, histone deacetylase; IUDR, 5-iodo-2’deoxyuridine; TK, tyrosine kinase; UCN-01, 7-hydroxystaurosporine.
Radioprotectors for WBRT.
A proportion of patients receiving WBRT suffer from progressive, permanent cognitive impairment. A recent clinical trial demonstrated a reduction in cognitive function in patients with NSCLC who were treated with WBRT, as assessed by a specific memory test115. The deleterious effects of WBRT on cognition have limited its use, particularly in cancer patients who have stable systemic disease and an expected prolonged survival period. However, WBRT-induced cognitive decline is difficult to measure as it involves patient function at baseline (already deteriorated by the contributions of brain metastases and ‘chemobrain’ from systemic therapy), the adequacy of testing methods and the variety of drugs administered to patients.
A growing body of evidence suggests that chronic oxidative stress and inflammation have a role in cognitive decline116. Irradiating the adult rodent brain leads to neuroinflammation, increased oxidative stress117,118, activation of microglia119–121, and a chronic, progressive loss of both hippocampal-dependent and non-hippocampal-dependent cognitive function. A stem cell population in the vicinity of the hippocampus could be responsible for producing mature neurons, and, following radiation injury, the inflammatory process may alter the neurogenic fate of these stem cells to a more gliogenic fate, thereby also causing memory deficits122. The cognitive effects of WBRT have been modelled in non-cancer-bearing animals. Adult rats were treated with WBRT, and cognitive function was quantified over the next year using a battery of tests, including regular and water mazes, as well as novel object recognition tests.
Relative cognitive function was 73 ±6% at 12 weeks post-WBRT and decreased to 45 ±4% and 14 ±4% at 26 and 52 weeks post-WBRT, respectively, which is indicative of late, chronic and progressive cognitive impairment123.
Anti-inflammatory-based interventions have been hypothesized to prevent or ameliorate radiation-induced cognitive impairment. In non-tumour-bearing animals, the administration of pioglitazone (Actos; Takeda) (a peroxisome proliferator-activated receptor-γ (PPARγ) agonist that is prescribed for diabetes124), fenofibrate (Lipantil; Abbott Laboratories) (a PPARα agonist that is prescribed for hypercholesterolaemia and hypertriglyceridaemia125) or the angiotensin type 1 receptor (AGTR1) antagonist (AT1RA) L-158809 (an angiotensin-converting enzyme (ACE) inhibitor that is typically used to treat hypertension) significantly ameliorated WBRT-induced cognitive impairment126–128. These and similar studies suggest the intriguing hypothesis that some of the cognitive impairment that is associated with WBRT can be prevented using radioprotectors. Other potential radioprotectors tested in non-cancer brain diseases include melanocortins, erythropoietin, statins and antibiotics of the tetracycline and fluoroquinolone classes129. Studies in brain metastatic models are awaited in order to demonstrate the preservation of cognition, as well as the effects of the drug on metastatic colonization. Most animal models have been developed to produce brain lesions quickly, and this field will require new models permitting time for cognitive dysfunction to appear. Radiation-protection clinical trials would enrol newly diagnosed patients with brain metastasis who had an expected survival long enough to permit the development of cognitive sequelae; patients would be randomized to a protracted course of placebo or the investigational agent combined with WBRT. End points would be a decline in performance based on regularly administered cognitive tests, as well as quality of life, radiographic changes in brain lesions and patient survival.
Conclusions
Brain metastases cause physical and cognitive morbidities and limit the survival of cancer patients, particularly those with advanced melanoma, lung cancer and breast cancer. As chemotherapy improves for other cancer types, the incidence of brain metastases is likely to rise as a sanctuary site. WBRT has efficacy as a brain metastasis-preventive therapy. New leads into the radioprotection of the normal brain to prevent cognitive loss from WBRT, and radiosensitization of tumour kill by SRS, may bring radiation therapy into safer, more effective use. Drug development can attack the problem of brain metastasis by identifying mechanistic molecular pathways, validating brain-permeable inhibitors and clinically testing them in combination with systemic therapy and/or radiation. The currently available preclinical data suggest that chemotherapeutic drugs may be most effective in the prevention of brain metastases rather than the shrinkage of established lesions, which will require new trial designs. Comprehensive evaluations of patient cognition and quality of life will be essential to meaningfully progress.
Acknowledegements
This work was supported by the Intramural Program, Center for Cancer Research, US National Cancer Institute, and from the Center of Excellence grant W81XWH-062–0033 from the US Department of Defense Breast Cancer Research Program. The authors regret that space restrictions did not permit all citations to be included.
Footnotes
Competing interests statement The authors declare competing financial interests. See Web version for details.
FURTHER INFORMATION
Patricia S. Steeg’s homepage:
Parenchymal metastases
Secondary tumour growth in the essential and distinctive tissue of the brain
Leptomeningeal metastases
Secondary tumour growth in the linings of the brain
Cranial neuropathies
Abnormal function (either sensory or motor) of one of the 12 cranial nerves
Stereotactic radiosurgery
Radiation therapy in which multiple convergent beams of high energy X-rays, γ-rays or protons are delivered to a discrete lesion in the brain
Astrocytes
Brain cells that form a physical and metabolic support system for nerves while releasing communicative transmitters. When activated, astrocytes produce glial fibrillary acid protein intermediate filaments and shield neurons from damage
Iron oxide particles
In magnetic resonance imaging, these supramagnetic particles generate a region emitting no radiofrequency signal, known as a signal void
Temozolomide
A brain-permeable chemotherapeutic with alkylating activity
Partial response
At least a 30% decrease in the sum of diameters of target lesions, taking as reference the baseline sum diameters
Stable disease
Neither sufficient shrinkage to qualify for partial response nor sufficient increase to qualify for progressive disease, taking as reference the smallest sum diameters while on study
Disease progression
At least a 20% increase in the sum of diameters of target lesions, taking as reference the smallest sum on study (this includes the baseline sum). In addition to the relative increase of 20%, the sum must also demonstrate an absolute increase of at least 5 mm
Facilitated diffusion
The spontaneous passage of molecules or ions across a biological membrane passing through specific transmembrane integral proteins
Epothilones
A new class of microtubule-active drugs
Nomogram
A form of line chart showing scales for the variables involved in a particular formula so that corresponding values for each variable lie in a straight line intersecting all the scales
Performance status
A measure of a patient’s well-being defined as the amount of normal activity that the patient can maintain
References
- 1.Yamanaka R Medical management of brain metastases from lung cancer (Review). Oncol. Rep. 22, 1269–1276 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Oh Y et al. Number of metastatic sites is a strong predictor of survival in paitents with nonsmall cell lung cancer with or without brain metastases. Cancer 115, 2930–2938 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Lin N, Bellon J & Winer E CNS metastases in breast cancer. J. Clin. Oncol. 22, 3608–3617 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Lin NU & Winer EP Brain metastases: the HER2 paradigm. Clin. Cancer Res. 13, 1648–1655 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Weil R, Palmieri D, Bronder J, Stark A & Steeg P Breast cancer metastasis to the central nervous system. Am. J. Pathol. 167, 913–920 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bendell J et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer 97, 2972–2977 (2003). [DOI] [PubMed] [Google Scholar]
- 7.Lin N et al. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer. High incidence of central nervous system metastases. Cancer 113, 2638–2645 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsukada Y, Fouad A, Pickren JW & Lane W W Central nervous system metastasis from breast carcinoma. Autopsy study. Cancer 52, 2349–2354 (1983). [DOI] [PubMed] [Google Scholar]
- 9.Miller K et al. Occult central nervous system involvement in patients with metastatic breast cancer: prevalence, predictive factors and impact on overall survival. Ann. Oncol. 14, 1072–1077 (2003).This paper reports the frequency of undiagnosed brain metastases in a modern chemotherapeutic setting. [DOI] [PubMed] [Google Scholar]
- 10.McWilliams R et al. Melanoma-induced brain metastases. Expert Rev. Anticancer Ther. 8, 743–755 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Mattieu A et al. Development of a chemoresistant orthotopic human nonsmall cell lung carcinoma model in nude mice. Cancer 101, 1908–1918 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z, Hatori T & Nonaka H An experimental model of brain metastasis of lung carcinoma. Neuropathology 28, 24–28 (2008). [DOI] [PubMed] [Google Scholar]
- 13.Kienast Y et al. Real-time imaging reveals the single steps in brain metastasis formation. Nature Med. 16, 116–122 (2010).This paper used imaging to detail the process of brain colonization by experimental metastases. [DOI] [PubMed] [Google Scholar]
- 14.Yoneda T, Williams P, Hiraga T, Niewolna M & Nishimura R A bone seeking clone exhibits different biological properties from the MDA-MB-231 parental human breast cancer cells and a brain-seeking cloe in vivo and in vitro. J. Bone Miner. Res. 16, 1486–1495 (2001). [DOI] [PubMed] [Google Scholar]
- 15.Lockman P et al. Heterogeneous blood-brain barrier permeability determines drug efficacy in mouse brain metastases of breast cancer. Clin. Cancer Res. 16, 5662–5678 (2010).This paper quantifies the heterogeneous permeability of experimental brain metastases and shows that only ~10% of lesions demonstrate sufficient chemotherapeutic permeability to produce a cytotoxic response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gril B et al. Pazopanib reveals a role for tumor cell B-Raf in the prevention of breast cancer brain metastasis. Clin. Cancer Res. 17, 142–153 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bos P et al. Genes that mediate breast cancer metastasis to the brain. Nature 459, 1005–1010 (2009).A paper detailing the molecular characterization of breast cancer brain metastases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Price JE, Polyzos A, Zhang RD & Daniels LM Tumorigenicity and metastasis of human breast carcinoma cell lines in nude mice. Cancer Res. 50, 717–721 (1990). [PubMed] [Google Scholar]
- 19.Rye P et al. Brain metastasis model in athymic nude mice using a novel MUC1-secreting human breast-cancer cell line, MA11. Int. J. Cancer 68, 682–687 (1996). [DOI] [PubMed] [Google Scholar]
- 20.Carbonell W, Ansorge O, Sibson N & Muschel R The vascular basement membrane as “soil” in brain metastasis. PLoS ONE 4, e5857 (2009).This paper traces the colonization of the brain by brain-tropic metastatic cells and validates the contribution of integrins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leenders W et al. Antiangiogenic therapy of cerebral melanoma metastases results in sustained tumor progression via vessel co-option. Clin. Cancer Res. 10, 6222–6230 (2004).This is an analysis of vascular co-option versus angiogenesis in experimental brain metastasis. [DOI] [PubMed] [Google Scholar]
- 22.Cranmer LD, Trevor KT, Bandlamuri S & Hersh EM Rodent models of brain metastasis in melanoma. Melanoma Res. 15, 325–356 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Zhang C, Zhang F, Tsan R & Fidler I Transforming growth factor (32 is a molecular determinant for site specific melanoma metastasis in the brain. Cancer Res. 69, 828–835 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang F-J et al. Molecular basis of the critical role of suppressor of cytokine signaling-1 in melanoma brain metastasis. Cancer Res. 68, 9634–9642 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin J et al. Noninvasive imaging of the functional effects of anti-VEGF therapy on tumor cell extravasation and regional blood volume in an experimental brain metastasis model. Clin. Exp. Metastasis 26, 403–414 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kruger A et al. Host TIMP-1 overexpression confers resistance to experimental brain metastasis of a fibrosarcoma cell line. Oncogene 16, 2419–2423 (1998). [DOI] [PubMed] [Google Scholar]
- 27.Maillard C et al. Reduction of brain metastases in plasminogen activator inhibitor-1 deficient mice with transgenic ocular tumors. Carcinogenesis 29, 2236–2242 (2008). [DOI] [PubMed] [Google Scholar]
- 28.Cruz-Munoz W, Man S, Xu P & Kerbel R Development of a preclinical model of spontaneous human melanoma central nervous system metastasis. Cancer Res. 68, 4500–4505 (2008). [DOI] [PubMed] [Google Scholar]
- 29.Fitzgerald D et al. Reactive glia are recruited by highly proliferative brain metastases of breast cancer and promote tumor cell colonization. Clin. Exp. Metast. 25, 799–810 (2008).This paper validates the relevance of preclinical experimental metastasis models to resected human tissues. It describes a functional interaction between tumour cells and neuroinflammatory cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paget S The distribution of secondary growths in cancer of the breast. Lancet 1, 99–101 (1889). [PubMed] [Google Scholar]
- 31.Lorger M & Felding-Habermann B Capturing changes in the brain microenvironment during intial steps of breast cancer brain metastasis. Am. J. Pathol. 176, 2958–2971 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang M & Olsson Y Hematogenous metastases of the human brain - characteristics of peritumoral brain changes: a review. J. Neurooncol. 35, 81–89 (1997). [DOI] [PubMed] [Google Scholar]
- 33.Seike T et al. Interaction between lung cancer cells and astrocytes via specific inflammatory cytokines in the microenvironment of brain metastasis. Clin. Exp. Metastasis 28, 13–25 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin Q et al. Reactive astrocytes protect melanoma cells from chemotherapy by sequestering intracellular calcium through gap junction communication channels. Neoplasia 12, 748–754 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pukrop R et al. Microglia promote the colonization of brain tissue by breast cancer cells in a Wnt-dependent way. Glia 58, 1477–1489 (2010). [DOI] [PubMed] [Google Scholar]
- 36.Salgado K, Toscani N, Silva L, Hilbig A & Barbosa-Coutinho L Immunoexpression of endoglin in brain metastasis secondary to malignant melanoma: evaluation of angiogenesis and comparison with brain metastasis secondary to breast and lung carcinomas. Clin. Exp. Metastasis 24, 403–410 (2007). [DOI] [PubMed] [Google Scholar]
- 37.Leenders W et al. Vascular endothelial growth factor-A determines detectability of experimental melanoma brain metastasis in GD-DTPA-enhanced MRI. Int. J. Cancer 105, 437–443 (2003). [DOI] [PubMed] [Google Scholar]
- 38.Kim L, Huang S, Lu W, Lev DC & Price J Vascular endothelial growth factor expression promotes the growth of breast cancer brain metastases in nude mice. Clin. Exp. Metastasis 21, 107–118 (2004). [DOI] [PubMed] [Google Scholar]
- 39.Heyn C et al. In vivo magnetic resonance imaging of single cells in mouse brains with optical validation. Magn. Reson. Med. 55, 23–29 (2006).A study showing that dormant tumour cells exist in the brain using in vivo imaging and post-mortem microscopy. [DOI] [PubMed] [Google Scholar]
- 40.Silva LD et al. HER3 and downstream pathways are involved in colonization of brain metastases from breast cancer. Breast Cancer Res. 12, 1–13 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun M et al. HER family receptor abnormalities in lung cancer brain metastases and corresponding primary tumors. Clin. Cancer Res. 15, 4829–4837 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koo J & Kim S EGFR and HER-2 status of non-small cell lung cancer brain metastasis and corresponding primary tumor. Neoplasma 58, 27–34 (2011). [DOI] [PubMed] [Google Scholar]
- 43.Milas I et al. Epidermal growth factor receptor, cyclooxygenase-2, and BAX expression in the primary non-small cell lung cancer and brain metastases. Clin. Cancer Res. 9, 1070–1076 (2003). [PubMed] [Google Scholar]
- 44.Gaedcke J et al. Predominance of the basal type and HER-2/neu type in brain metastasis from breast cancer. Mod. Pathol. 20, 864–870 (2007). [DOI] [PubMed] [Google Scholar]
- 45.Wu P-F et al. O6-Methylguanine-DNA methyltransferase expression and prognostic value in brain metastases of lung cancers. Lung Cancer 68, 484–490 (2010). [DOI] [PubMed] [Google Scholar]
- 46.Gomez-Roca C et al. Differential expression of biomarkers in primary non-small cell lung cancer and metastatic sites. J. Thorac. Oncol. 4, 1212–1220 (2009). [DOI] [PubMed] [Google Scholar]
- 47.Mehrotra J et al. Very high frequency of hypermethylated genes in breast cancer metastasis to bone, brain and lung. Clin. Cancer Res. 10, 3104–3109 (2004). [DOI] [PubMed] [Google Scholar]
- 48.Ding L et al. Genome remodeling in a basal-like breast cancer metastasis and xenograft. Nature 464, 999–1005 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stark A, Tongers K, Maass N, Mehdom H & Held-Feidt J Reduced metastasis suppressor gene mRNA expression in breast cancer brain metastases. J. Cancer Res. Clin. Oncol. 131, 191–198 (2005). [DOI] [PubMed] [Google Scholar]
- 50.Stark A et al. Reduced mRNA and protein expression of BCL-2 versus decreased mRNA and increased protein expression of BAX in breast cancer brain metastases: a real-time PCR and immunohistochemical evaluation. NuerologicalRes. 28, 787–793 (2006). [DOI] [PubMed] [Google Scholar]
- 51.Veenendaal L et al. Differential notch and TGFβ signaling in primary colorectal tumors and their corresponding metastases. Cell. Oncol. 30, 1–11 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palmieri D et al. Analyses of resected human brain metastases of breast cancer reveal the association between up-regulation of hexokinase 2 and poor prognosis. Mol. Cancer Res. 7, 1438–1445 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xie TX et al. Activation of stat3 in human melanoma promotes brain metastasis. Cancer Res. 66, 3188–3196 (2006). [DOI] [PubMed] [Google Scholar]
- 54.Palmieri D et al. Her-2 overexpression increases the metastatic outgrowth of breast cancer cells in the brain. Cancer Res. 67, 4190–4198 (2007). [DOI] [PubMed] [Google Scholar]
- 55.Navab R et al. Co-overexpression of Met and Hepatocyte growth factor promotes systemic metastasis in NCI-H460 non-small cell lung carcinoma cells. Neoplasia 11, 1292–1300 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nguyen D et al. WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell 138, 51–62 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang L, Sullivan P, Goodman J, Gunaratne P & Marchetti D MicroRNA-1258 suppresses breast cancer brain metastasis by targeting heparanase. Cancer Res. 71, 645–654 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cocconi G et al. Combination therapy with platinum and etoposide of brain metastases from breast carcinoma. Cancer Invest. 8, 327–334 (1990). [DOI] [PubMed] [Google Scholar]
- 59.Boogerd W, Dalesio O, Bais EM & van der Sande JJ Response of brain metastases from breast cancer to systemic chemotherapy. Cancer 69, 972–980 (1992). [DOI] [PubMed] [Google Scholar]
- 60.Rosner D, Nemoto T & Lane WW Chemotherapy induces regression of brain metastases in breast carcinoma. Cancer 58, 832–839 (1986). [DOI] [PubMed] [Google Scholar]
- 61.Oberhoff C et al. Topotecan chemotherapy in patients with breast cancer and brain metastases: results of a pilot study. Onkologie 24, 256–260 (2001). [DOI] [PubMed] [Google Scholar]
- 62.Kurt M, Aksoy S, Hayran M & Guler N A retrospective review of breast cancer patients with central nervous system metastases treated with capecitabine. J. Clin. Oncol. Abstr. 25, 1098 (2007). [Google Scholar]
- 63.Lin N et al. Phase II trial of lapatinib for brain metastases in patients with HER2+ breast cancer. J. Clin. Oncol. Abstr 24, 503 (2006). [Google Scholar]
- 64.Lin N et al. Multicenter Phase II study of lapatinib in patients with brain metastases from HER-2 positive breast cancer. Clin. Cancer Res. 15, 1452–1459 (2009). [DOI] [PubMed] [Google Scholar]
- 65.Quirt I et al. Temozolomide for the treatment of metastatic melanoma. Curr. Oncol. 14, 27–33 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ekenel M, Hormigo A, Peak S, DeAngelis L & Abrey L Capecitabine therapy of central nervous system metastases from breast cancer. J. Neurooncol. 85, 223–227 (2007). [DOI] [PubMed] [Google Scholar]
- 67.Rivera E et al. Phase I study of capecitabine in combination with temozolomide in the treatment of patients with brain metastases of breast cancer. Cancer 107, 1348–1354 (2006). [DOI] [PubMed] [Google Scholar]
- 68.Mehta M et al. The role of chemotherapy in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J. Neurooncol. 96, 71–83 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ceresoli G et al. Gefitinib in patients with brain metastases from non-small cell lung cancer: a prospective trial. Ann. Oncol. 15, 1042–1047 (2004). [DOI] [PubMed] [Google Scholar]
- 70.Wu C et al. Gefitinib as palliative therapy for lung adenocarcinoma metastatic to the brain. Lung Cancer 57, 359–364 (2007). [DOI] [PubMed] [Google Scholar]
- 71.Togashi Y et al. Cerebrospinal fluid concentration of erlotinib and its active metabolite OSI-420 in patients with central nervous system metastases of non-small cell lung cancer. J. Thorac. Oncol. 5, 950–955 (2010). [DOI] [PubMed] [Google Scholar]
- 72.Omuro AM et al. High incidence of disease recurrence in the brain and leptomeninges in patients with nonsmall cell lung carcinoma after response to gefitinib. Cancer 103, 2344–2348 (2005). [DOI] [PubMed] [Google Scholar]
- 73.Bria E et al. Cardiotoxicity and incidence of brain metastases after adjuvant trastuzumab for early breast cancer: the dark side of the moon? A meta-analysis of the randomized trials. Breast Cancer Res. Treat. 109, 231–239 (2008). [DOI] [PubMed] [Google Scholar]
- 74.Stemmler H-J et al. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of the blood-brain barrier. Anticancer Drugs 18, 23–28 (2007). [DOI] [PubMed] [Google Scholar]
- 75.Polli J et al. The role of efflux and uptake transporters in N-{3-chloro-4-[(3-fluorobenzyl)oxy] phenyl}−6-[5-({[2-(methylsulfonyl)ethyl]amino} methyl)-2-furyl]-4-quinazolinamine (GWS572016, Lapatinib) disposition and drug interactions. Drug Metab. Dispos. 36, 695–701 (2008). [DOI] [PubMed] [Google Scholar]
- 76.Lien EA, Wester K, Lønning PE, Solheim E & Ueland PM Distribution of tamoxifen and metabolites into brain tissue and brain metastases in breast cancer patients. Br. J. Cancer 63, 641–645 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Socinski M et al. Safety of bevicizumab in patients with non-small-cell lung cancer and brain metastases. J. Clin. Oncol. 27, 5255–5261 (2009). [DOI] [PubMed] [Google Scholar]
- 78.Stadler W et al. Safety and efficacy results of the advanced renal cell carcinoma sorafenib expanded access program in north America. Cancer 116, 1272–1280 (2010). [DOI] [PubMed] [Google Scholar]
- 79.Pajouhesh H & Lenz GR Medicinal chemical properties of successful central nervous system drugs. NeuroRx 2, 541–553 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ohtsuki S & Terasaki T Contribution of carrier-mediated transport systems to the blood-brain barrier as a supporting and protecting interface for the brain; importance for CNS drug discovery and development. Pharm. Res. 24, 1745–1758 (2007). [DOI] [PubMed] [Google Scholar]
- 81.Szakacs G, Paterson J, Ludwig J, Booth-Genthe C & Gottesman M Targeting multidrug resistance in cancer. Nature Rev. Drug Discov. 5, 219–234 (2006). [DOI] [PubMed] [Google Scholar]
- 82.Noguchi K, Katayama K, Mitsuhashi J & Sugimoto Y Functions of the breast cancer resistance protein (BCRP/ABCG2) in chemotherapy. Adv. Drug Deliv. Rev. 61, 26–33 (2009). [DOI] [PubMed] [Google Scholar]
- 83.Lagas J et al. Breast cancer resistance protein and P-glycoprotein limit sorafenib brain accumulation. Mol. CancerTher. 9, 319–326 (2010). [DOI] [PubMed] [Google Scholar]
- 84.Agarwal S, Sane R, Gallardo JL, Ohlfest JR & Elmquist WF Distribution of gefitinib to the brain is limited by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2)-mediated active efflux. J. Pharmacol. Exp. Ther. 334, 147–155 (2010).This paper provides an excellent demonstration of roles of multiple BBB efflux transporters in restricting the brain distribution of chemotherapeutic drugs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Armulik A et al. Pericytes regulate the blood-brain barrier. Nature 468, 557–561 (2010). [DOI] [PubMed] [Google Scholar]
- 86.Daneman R, Zhou L, Kebede AA & Barres BA Ericytes are required for blood-brain barrier integrity during embryogenesis. Nature 468, 562–566 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ahmad A, Gassmann M & Ogunshola O Maintaining blood-brain barrier integrity: pericytes perform better than astrocytes during prolonged oxygen deprivation. J. Cell. Physiol. 218, 612–622 (2009). [DOI] [PubMed] [Google Scholar]
- 88.Palmieri D et al. Vorinostat inhibits brain metastatic colonization in a model of triple-negative breast cancer. Clin. Cancer Res. 15, 6148–6157 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Luu T et al. A Phase II trial of vorinostat (Suberoylanilide hydroxamic acid) in metastatic breast cancer: a California Cancer Consortium study. Clin. Cancer Res. 14, 7138–7142 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ramalingam S et al. Carboplatin and paclitaxel in combination with either vorinostat or placebo for first-line therapy of advanced non-small-cell lung cancer. J. Clin. Oncol. 28, 56–62 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Baschnagel A et al. Vorinostat enhances the radiosensitivity of a breast cancer brain metastatic cell line grown in vitro and as intracranial xenografts. Mol. Cancer Ther. 8, 1589–1595 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gril B et al. Effect of lapatinib on the outgrowth of metastatic breast cancer cells to the brain. J. Natl. Cancer Inst. 100, 1092–1103 (2008).This is the first demonstration that a molecular therapeutic can prevent ERRB2+ experimental brain metastases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Emanuel S et al. Cellular and in vivo activity of JNJ-28871063, a nonquinazoline pan-ErbB kinase inhibitor that crosses the blood-brain barrier and displays efficacy against intracranial tumors. Mol. Pharmacol. 73, 338–348 (2008). [DOI] [PubMed] [Google Scholar]
- 94.Kong L-Y et al. A novel inhibitor of signal transducers and activators of transcription 3 activation is efficaceous against established central nervous system melanoma and inhibits regulatory T cells. Clin. Cancer Res. 14, 5759–5768 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hoffmann J et al. Sagopilone crosses the blood-brain barrier in vivo to inhibit brain tumor growth and metastases. Neuro-oncology 11, 158–166 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ozduman K, Wollman G, Piepmeier J & van den Pol AN Systemic vesicular stomatitus virus selectively destroys multifocal glioma and metastatic carcinoma in the brain. J. Neursci. 28, 1882–1893 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang W et al. Reovirus as an experimental therapeutic for brain and leptomeningeal metastases from breast cancer. Gene Therapy 11, 1579–1590 (2004). [DOI] [PubMed] [Google Scholar]
- 98.Massard C et al. Incidence of brain metastases in renal cell carcinoma treated with sorafenib. Ann. Oncol. 21, 1027–1031 (2010). [DOI] [PubMed] [Google Scholar]
- 99.Cameron D et al. A Phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res. Treat. 112, 533–543 (2008). [DOI] [PubMed] [Google Scholar]
- 100.Heon S et al. Development of central nervous system metastases in patients with advanced non-small cell lung cancer and somatic EGFR mutations treated with gefintib or erlotinib. Clin. Cancer Res. 16, 5873–5882 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Grinberg-Rashi H et al. The expression of three genes in primary non-small cell lung cancer is associated with metastatic spread to the brain. Clin. Cancer Res. 15, 1755–1761 (2009). [DOI] [PubMed] [Google Scholar]
- 102.Grasslin O et al. Nomogram to predict subsequent brain metastasis in patients with metastatic breast cancer. J. Clin. Oncol. 28, 2032–2037 (2010). [DOI] [PubMed] [Google Scholar]
- 103.Sperduto P et al. A graded prosnostic assessment (GPA) for women with breast cancer and brain metastasssses. J. Clin. Oncol. Suppl. Abstr. 28 1028 (2010). [Google Scholar]
- 104.Thomas F et al. Uptake of ANG1005, a novel paclitaxel derivative, through the blood-brain barrier into brain and experimental brain metastases of breast cancer. Pharm. Res. 26, 2486–2494 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hu J et al. Phosphodiesterase type 5 inhibitors increase herceptin transport and treatment efficacy in mouse metastatic brain tumor models. PLoS ONE 5, e10108 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yuan H et al. Effects of fractionated radiation on the brain vasculature in a murine model: blood-brain barrier permeability, astrocyte proliferation, and ultrastructural changes. Int. J. Radiat. Oncol. Biol. Phys. 66, 860–866 (2006). [DOI] [PubMed] [Google Scholar]
- 107.Muldoon L et al. Chemotherapy delivery issues in central nervous system malignancy: a reality check. J. Clin. Oncol. 16, 2295–2305 (2007). [DOI] [PubMed] [Google Scholar]
- 108.Rosenberg A & Knox S Radiation sensitization with redox modulators: a promising approach. Int. J. Radiat. Oncol. Biol. Phys. 64, 343–354 (2006). [DOI] [PubMed] [Google Scholar]
- 109.Francis D, Richards G, Forouzannia A, Mehta M & Kuntia D Motexafin gadolinium: a novel radiosensitizer for brain tumors. Expert Opin. Pharmacother. 10, 2171–2180 (2009). [DOI] [PubMed] [Google Scholar]
- 110.Russo A et al. In vitro and in vivo radiosensitization of glioblastoma cells by the poly (ADP-Ribose) polymerase inhibitor E7016. Clin. Cancer Res. 15, 607–612 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Morgan M et al. Mechanism of radiosensitization by the Chk½ inhibitor AZD7762 involves abrogation of the G2 checkpoint and inhibition of homologous recombinational DNA repair. Cancer Res. 70, 4972–4981 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gerster K et al. Targeting polo-like kinase 1 enhances radiation efficacy for head-and-neck squamous cell carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 77, 253–260 (2010). [DOI] [PubMed] [Google Scholar]
- 113.Chung E et al. In vitro and in vivo radiosensitization with AZD6244 (ARRY-142886), an inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase ½ kinase. Clin. Cancer Res. 15, 3050–3057 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Camphausen K et al. Orthotopic growth of human glioma cells quantitatively and qualitatively influences radiation-induced changes in gene expression. Cancer Res. 65, 10389–10393 (2005). [DOI] [PubMed] [Google Scholar]
- 115.Chang E et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomized controlled trial. Lancet Oncol. 10, 1037–1044 (2009). [DOI] [PubMed] [Google Scholar]
- 116.Zhao W & Robbins M Inflammation and chronic oxidative stress in radiation-induced late normal tissue injury: therapeutic implications. Curr. Med. Chem. 16, 130–143 (2009). [DOI] [PubMed] [Google Scholar]
- 117.Fukuda H et al. Irradiation-induced progenitor cell death in the developing brain is resistant to erythropoietin treatment and caspase inhibition. Cell Death Differ. 11, 1166–1178 (2004). [DOI] [PubMed] [Google Scholar]
- 118.Limoli C et al. Cell-density-dependent regulation of neural precursor cells function. Proc. Natl Acad. Sci. USA 101, 16052–16057 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mildenberger M, Beach T, McGeer E & Ludgate C An animal model of prophylactic cranial irradiation: histologic effects at acute, early and delayed stages. Int. J. Radiat. Oncol. Biol. Phys. 18, 1051–1060 (1990). [DOI] [PubMed] [Google Scholar]
- 120.Monje M, Toda H & Palmer T Inflammatory blockade restores adult hippocampal neurogenesis. Science 302, 1760–1765 (2003). [DOI] [PubMed] [Google Scholar]
- 121.Schindler M, Forbes M, Robbins M & Riddle D Aging-dependent changes in the radiation response of the adult rat brain. Int. J. Radiat. Oncol. Biol. Phys. 70, 826–834 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Khuntia D, Brown P, Li J & Mehta M Whole-brain radiotherapy in the management of brain metastasis. J. Clin. Oncol. 24, 1295–1304 (2006). [DOI] [PubMed] [Google Scholar]
- 123.Robbins M et al. The AT1 receptor antagonist, L-158, 809, prevents or ameliorates factionated whole-brain irradiation-induced cognitive impairment. Int. J. Radiat. Oncol. Biol. Phys. 73, 499–505 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Clar C, Royle P & Waugh N Adding piaglitazone to insulin containing regimens in type 2 diabetes: systematic review and meta-analysis. PLoS ONE 4, e6112 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Abourbih S et al. Effect of bibrates on lipid profiles and cardiovascular outcomes: a systematic review. Am. J. Med. 122, 962.e1–962.e8 (2009). [DOI] [PubMed] [Google Scholar]
- 126.Zhao W et al. Administration of the peroxisomal proliferator-activated receptor (PPAR)g pioglitazone during fractionated brain irradiation prevents radiation-induced cognitive impariment. Int. J. Radiat. Oncol. Biol. Phys. 67, 6–9 (2007). [DOI] [PubMed] [Google Scholar]
- 127.Ramanan S et al. The PPARα agonist fenobibrate preserves hippocampal neurogenesis and inhibits microglial activation after whole-brain radiation. Int. J. Radiat. Oncol. Biol. Phys. 75, 870–877 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Atwood T et al. Quantitative magnetic resonance spectroscopy reveals a potential relationship between radiation-induced changes in rat brain metabolites and cognitive impairment. Radiat. Res. 168, 574–581 (2007). [DOI] [PubMed] [Google Scholar]
- 129.Kim K et al. High-throughput screening identifies two classes of antibiotics as radioprotectors: tetracyclines and fluoroquinolones. Clin. Cancer Res. 15, 7238–7245 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gleissner B & Chamberlain M Neoplastic meningitis. Lancet Neurol. 5, 443–451 (2006). [DOI] [PubMed] [Google Scholar]
- 131.Pedersen P-H et al. Leptomeningeal tissue: a barrier against brain tumor cell invasion. J. Natl Cancer Inst. 86, 1593–1599 (1994). [DOI] [PubMed] [Google Scholar]
- 132.Herrlinger U et al. Leptomeningeal metastasis: survival and prognostic factors in 155 patients. J. Neurol. Sci. 223, 167–178 (2004). [DOI] [PubMed] [Google Scholar]
- 133.Kesari S & Batchelor T Leptomeningeal metastases. Neurol. Clin. 21, 25–66 (2003). [DOI] [PubMed] [Google Scholar]
- 134.Borgelt B et al. Ultra-rapid high dose irradiation schedules for the palliation of brain metastases: final results of the first two studies by the Radiation Therapy Oncology Group. Int. J. Radiat. Oncol. Biol. Phys. 7, 1633–1638 (1981). [DOI] [PubMed] [Google Scholar]
- 135.Borgelt B et al. The palliation of brain metastases: final results of the first two studies by the radiation therapy oncology group. Int. J. Radiat. Oncol. Biol. Phys. 6, 1–9 (1980). [DOI] [PubMed] [Google Scholar]
- 136.Patil CG, Pricola K, Garg SK, Bryant A & Black KL Whole brain radiation therapy (WBRT) alone versus WBRT and radiosurgery for the treatment of brain metastases. Cochrane Database Syst. Rev. CD006121 (2010). [DOI] [PubMed] [Google Scholar]
- 137.Kalkanis S et al. The role of surgical resection in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J. Neurooncol. 96, 33–44 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Slotman B et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. New Engl. J. Med. 357, 664–672 (2007).This report establishes that prophylactic cranial irradiation can be used to prevent brain metastasis. [DOI] [PubMed] [Google Scholar]
- 139.Lester J, MacBeth F & Coles B Prophylactic cranial irradiation for preventing brain metastases in patients undergoing radical treatment for non-small-cell lung cancer: a Cochrane review. Int. J. Radiat. Oncol. Biol. Phys. 63, 690–694 (2005). [DOI] [PubMed] [Google Scholar]
- 140.Sanghavi S et al. Radiosurgery for patients with brain metastases: a multi-institutional analysis, stratified by RTOG recursive partitioning analysis method. Int. J. Radiat. Oncol. Biol. Phys. 51, 426–434 (2001). [DOI] [PubMed] [Google Scholar]
- 141.Petrovich Z, Yu C, Giannotta SL, O’Day S & Apuzzo ML Survival and pattern of failure in brain metastasis treated with stereotactic y knife radiosurgery. J. Neurosurg. 97, 499–506 (2002). [DOI] [PubMed] [Google Scholar]
- 142.Folkman J & Camphausen K What does radiotherapy do to endothelial cells? Science 293, 227–228 (2001). [DOI] [PubMed] [Google Scholar]


