Abstract
Background:
Anterior cruciate ligament (ACL) tears are common knee injuries. Despite undergoing extensive rehabilitation after ACL reconstruction (ACLR), many patients have persistent quadriceps muscle weakness that limits their successful return to play and are also at an increased risk of developing knee osteoarthritis (OA). Human growth hormone (HGH) has been shown to prevent muscle atrophy and weakness in various models of disuse and disease but has not been evaluated in patients undergoing ACLR.
Hypothesis:
Compared with placebo treatment, a 6-week perioperative treatment course of HGH would protect against muscle atrophy and weakness in patients undergoing ACLR.
Study Design:
Randomized controlled trial; Level of evidence, 2.
Methods:
A total of 19 male patients (aged 18-35 years) scheduled to undergo ACLR were randomly assigned to the placebo (n = 9) or HGH (n = 10) group. Patients began placebo or HGH treatment twice daily 1 week before surgery and continued through 5 weeks after surgery. Knee muscle strength and volume, patient-reported outcome scores, and circulating biomarkers were measured at several time points through 6 months after surgery. Mixed-effects models were used to evaluate differences between treatment groups and time points, and as this was a pilot study, significance was set at P < .10. The Cohen d was calculated to determine the effect size.
Results:
HGH was well-tolerated, and no differences in adverse events between the groups were observed. The HGH group had a 2.1-fold increase in circulating insulin-like growth factor 1 over the course of the treatment period (P < .05; d = 2.93). The primary outcome measure was knee extension strength, and HGH treatment increased normalized peak isokinetic knee extension torque by 29% compared with the placebo group (P = .05; d = 0.80). Matrix metalloproteinase–3 (MMP3), which was used as an indirect biomarker of cartilage degradation, was 36% lower in the HGH group (P = .05; d = −1.34). HGH did not appear to be associated with changes in muscle volume or patient-reported outcome scores.
Conclusion:
HGH improved quadriceps strength and reduced MMP3 levels in patients undergoing ACLR. On the basis of this pilot study, further trials to more comprehensively evaluate the ability of HGH to improve muscle function and potentially protect against OA in patients undergoing ACLR are warranted.
Registration:
NCT02420353 (ClinicalTrials.gov identifier)
Keywords: anterior cruciate ligament, muscle atrophy, human growth hormone, somatropin, orthobiologics
Anterior cruciate ligament (ACL) tears are among the most frequent traumatic knee injuries in sports medicine, with an average annual rate of surgical ACL reconstruction (ACLR) in the United States of 73.6 per 100,000 person-years.20 There have been advances in surgical repair techniques that have reduced graft failure rates, but many patients have persistent quadriceps muscle weakness after ACLR, with strength deficits as high as 40%, and are at an increased risk of developing osteoarthritis (OA) later in life.21,35 Muscle weakness after ACLR reduces the likelihood of return to play at the same level before the injury, increases the risk of reinjuries, and can contribute to the development of OA beyond the chondral and meniscal injuries themselves.22,33 Additionally, muscle weakness is associated with reduced long-term patient-reported function after ACL tears.10 Therefore, identifying a treatment option to prevent the loss of strength after ACLR could improve our ability to return patients to preinjury competition levels and limit the long-term development of OA.
Human growth hormone (HGH) is an important endocrine hormone that regulates metabolism and tissue growth and maintenance.19 One of the downstream effector molecules of HGH is insulin-like growth factor 1 (IGF-1), which is produced by various tissues, including skeletal muscle, in response to HGH.19 IGF-1 promotes muscle stem cell proliferation and activates Akt and the mTORC1 complex, which regulate muscle protein synthesis and can inhibit muscle protein catabolism.16,19 In animal models and studies of humans with disuse muscle atrophy, HGH treatment safely counteracted muscle wasting and preserved strength, function, and quality of life.5,14 IGF-1 also promotes cartilage matrix protein synthesis and prevents cartilage catabolism,11 which indicate a potentially chondroprotective role for IGF-1 in traumatic joint injuries. Given the substantial muscle weakness observed in many patients after ACLR, and the role of HGH and IGF-1 in protecting against weakness and dysfunction in various models of disuse atrophy, we conducted a randomized, placebo-controlled, pilot clinical trial in which patients undergoing ACLR were administered either HGH or placebo for a 6-week period beginning 1 week before surgery. We hypothesized that, compared with placebo treatment, a 6-week perioperative course of HGH would protect against muscle weakness and atrophy in patients undergoing ACLR. The overall objectives of the study were to evaluate if HGH would be safely tolerated in closely monitored patients undergoing ACLR and, if so, to collect pilot and early efficacy data to determine if subsequent larger clinical trials are warranted.
METHODS
Participants
This study was approved by the University of Michigan Medical School Institutional Review Board, and an Investigational New Drug Exemption was granted by the United States Food and Drug Administration (IND 123189). This study is listed on ClinicalTrials.gov (NCT02420353) and followed the Declaration of Helsinki. Inclusion and exclusion criteria are shown in Table 1, and the CONSORT (Consolidated Standards of Reporting Trials) diagram is presented in Figure 1A. Patients underwent blood work screening approximately 4 to 6 weeks before surgery, which included a complete blood count with automated differential, metabolic panel, and lipid panel as well as measurements of hemoglobin A1c, high-sensitivity C-reactive protein (hs-CRP), and IGF-1. Patients with laboratory values within clinically acceptable guidelines were eligible for study enrollment. Then, approximately 2 weeks before surgery (range, 1.1-5.4 weeks), baseline patient-reported outcome measures, strength testing, and magnetic resonance imaging (MRI) were performed before the administration of HGH or placebo. After ACLR, patients completed 6 postoperative visits, which occurred approximately 1 week (range, 0.4-1.1 weeks), 2 weeks (range, 1.7-2.3 weeks), 5 weeks (range, 4.4-6.4 weeks), 12 weeks (range, 11.9-13.1 weeks), 18 weeks (range, 16.9-19.3 weeks), and 26 weeks (range, 25.3-28.1 weeks) after surgery (Figure 1B).
TABLE 1.
Inclusion and Exclusion Criteriaa
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| • Male sex • Age 18-35 years • Unilateral complete ACL tear with or without a meniscal tear that occurred within 6 months of study enrollment • Consented to undergo ACLR • Agreed to undergo supervised postoperative rehabilitation at the University of Michigan MedSport Clinic |
• Revision ACLR • Previous injury to the involved knee • Allergy to HGH • Body mass index <20 or >35 kg/m2 • Systolic blood pressure >140 mm Hg • Diastolic blood pressure >90 mm Hg • Resting heart rate >110 or <40 beats per minute at screening • Growth disorder of skeleton or connective tissue • Type 1 or 2 diabetes mellitus • Carpal tunnel syndrome or trigger finger • Myopathy, cancer, endocrine disorder, or rheumatological disorder • Athletes who participate in sports organizations that prohibit the use of HGH |
ACL, anterior cruciate ligament; ACLR, anterior cruciate ligament reconstruction; HGH, human growth hormone.
Figure 1.
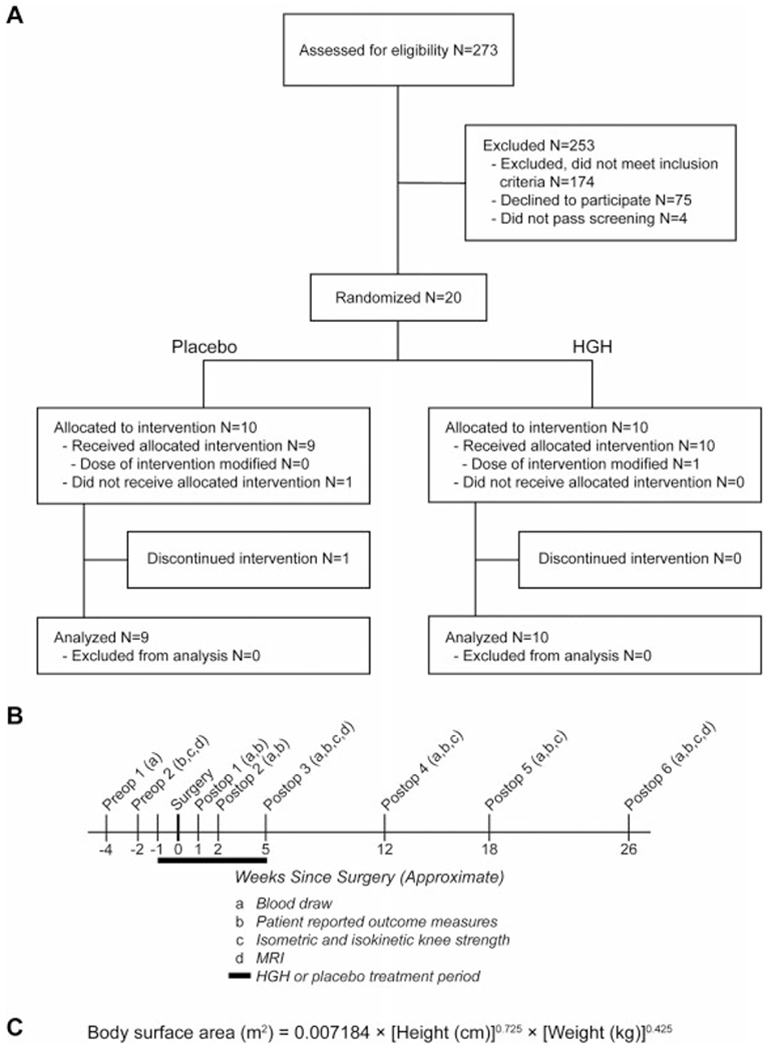
Study overview. (A) CONSORT (Consolidated Standards of Reporting Trials) diagram. (B) Approximate timeline of study events. (C) DuBois formula used to calculate the body surface area. HGH, human growth hormone; MRI, magnetic resonance imaging.
A 3-member safety committee, composed of a sports medicine physician (T.M.A.), a sports medicine orthopaedic surgeon (J.E.C.), and an endocrinologist (A.L.B.), oversaw the safety of the study and reviewed interim progress reports and adverse events. The committee was blinded to participant allocation. No specific rules were established that dictated stopping the trial. The committee was charged with considering the safety profile of HGH and the magnitude of effect, which was unknown in this patient population, as well as the objective of collecting enough data to design further clinical trials evaluating HGH in patients undergoing ACLR. A clinical epidemiologist (J.P.G.) was available for committee consultation.
To provide normative values for myostatin, matrix metalloproteinase–3 (MMP3), and hyaluronic acid (HA) biomarkers, we recruited an additional group of 9 age-matched healthy control participants without ACL tears (mean age, 27 years [range, 21-35 years] mean body mass index, 22.5 kg/m2 [range, 20.5-26.6 kg/m2]) using the same exclusion and inclusion criteria, except that participants were excluded if they had a previous joint injury. This portion of the study was approved by the Hospital for Special Surgery Institutional Review Board.
Study Group Assignment
Patients who passed safety screening and consented to enter the study were randomized to either the HGH or the placebo group. Using a random number generator, a block randomized assignment schedule that maintained equal group sizes was created before the start of the study.
HGH and Placebo Administration
Somatropin (Humatrope; NDC 0002-7335-11; Eli Lilly), which is HGH of recombinant DNA origin, or bacteriostatic saline, which served as the placebo (NDC 00409-1966-12; McKesson), was prepared by the University of Michigan Investigational Drug Service pharmacy to be doubly blinded to the patient and to study investigators. Patients underwent a training session and were instructed to deliver their dispensed medication via a subcutaneous injection into their abdominal area using an insulin syringe with a 31-gauge needle (BD). The dose was 0.5 mg of HGH per meter-square of body surface area (Figure 1C), twice daily, as a previous study used this same dose to significantly increase IGF-1 levels.40 This dose of HGH was approximately one-third of the dose that was shown to improve muscle function in patients with HIV-associated muscle wasting.9 Patients were asked to complete a log documenting administration. Treatment with HGH or placebo began 1 week before surgery and continued through 5 weeks postoperatively.
Surgical Reconstruction
Surgical reconstruction was performed by a fellowship-trained, high-volume ACL sports medicine orthopaedic surgeon (A.B.). Single-bundle anatomic reconstruction with a patellar tendon or hamstring tendon autograft was performed to recapitulate the native ligament footprint and ligament obliquity. A central-third patellar tendon or quadrupled semitendinosus and gracilis graft was harvested in the usual fashion. Routine arthroscopic surgery was performed, and a medial portal with an independent drilling technique was utilized for anatomic femoral socket preparation in all cases. All grafts were tensioned and fixed in full extension. Longitudinal meniscal tears were repaired if possible in all cases; tears in the white-white zone or with irreparable patterns were partially resected. Unstable partial-thickness chondral flaps were debrided; no full-thickness defects requiring marrow stimulation or autograft transplantation were present in this study.
Rehabilitation
Patients underwent postoperative rehabilitation at a single sports medicine clinic with a standardized rehabilitation protocol and the same rehabilitation staff to allow additional ad hoc safety monitoring between study visits. The program followed the general principles of the MOON Group recommendations42 of early return to full extension and early quadriceps strengthening. Rehabilitation began with quadriceps sets, straight-leg raises, ankle pumps, heel slides, and partial weightbearing up to 25% in the first week. These exercises continued into the second and third weeks, with the addition of electrical stimulation for muscle re-education, leg presses, core and hip exercises, stationary bicycling, and progression to 50% weightbearing. From weeks 4 to 12, additional closed kinetic chain resistance exercises were added, and patients progressed to full weightbearing and open kinetic chain exercises. From weeks 12 to 20, exercises increased in load and complexity, and patients progressed to a jogging program. Agility and plyometric training began at week 20 and continued in complexity and intensity based on passing functional tests, demonstrating appropriate gait and motor control.
Hematology and Blood Chemistry
Blood was drawn from the antecubital vein into a K2-EDTA, serum separator, or heparin Vacutainer tube (BD). Plasma or serum was prepared from whole blood, and samples that were not analyzed on the same day were stored at −80°C. Assays were performed by the University of Michigan Department of Pathology following clinical standard of care guidelines, with the exception of myostatin, MMP3, and HA, which were not available as clinical assays. Complete blood counts were measured in an XN-9000 system (Sysmex). Comprehensive metabolic panels, lipid panels, and hs-CRP were measured in an ADVIA 1800 system (Siemens). Hemoglobin A1c was determined in plasma samples using a G8 HPLC analyzer (Tosoh Bioscience). IGF-1 was measured from serum using an IMMULITE 2000 system (Siemens). Clinical normative values used for patient care were determined by the University of Michigan Department of Pathology, with the exception of published age-adjusted reference values for IGF-1.4 Myostatin (as a muscle atrophy biomarker) as well as MMP3 and HA (as cartilage biomarkers) were measured from serum (MMP3) or plasma (myostatin and HA) using enzyme-linked immunosorbent assays (ELISAs) (R&D Systems) as described29,30 and following manufacturer recommendations.
Some participants at the first and second postoperative visits exceeded the hs-CRP upper assay limit of 20 mg/L (first postoperative visit: n = 2 for placebo, n = 5 for HGH; second postoperative visit: n = 0 for placebo, n = 1 for HGH). These participants were recorded as 20 mg/L, and therefore, values at these visits underestimate actual values. All other values were within the calibration ranges of assays.
Magnetic Resonance Imaging
MRI to quantify muscle volume was performed using the same Ingenia 3.0-T MRI scanner (Philips). Axial mDIXON Quant sequences were conducted from just distal to the knee joint to the hip joint, and quadriceps and hamstring fat-cleared muscle volumes were calculated using AMRA Profiler software (AMRA Medical), as described,26 in a blinded manner. We measured absolute muscle volumes and also normalized the volume of the injured leg’s muscle group to the volume of the muscle group of the uninjured leg before surgery, as we believed that this value was most representative of the preinjury value of the injured leg. From the 57 scans analyzed, 9 had signal quality issues that prevented a quantitative assessment and were excluded from analysis.
Muscle Strength
Isometric and isokinetic knee flexion and extension strength measurements were obtained with a System 3 dynamometer (Biodex).15,30 Isometric measurements were performed at 90° of knee flexion. Isokinetic measurements were performed at a speed of 60 deg/s from a range of 0° to 90° of knee flexion. In addition to absolute values of injured legs, normalized values were calculated by dividing the value from the injured leg by the value from the contralateral uninjured leg before surgery. For each measurement, the highest force from a series of 5 repetitions was used.
Patient-Reported Outcome Measures
The Veterans RAND 12-Item Health Survey (VR-12) was used to measure health-related quality of life.38 The International Knee Documentation Committee (IKDC) form2 and the Knee injury and Osteoarthritis Outcome Score (KOOS)36 were used to assess knee- and physical activity-related outcomes.
Statistical Analysis
As this is a pilot and safety study that is not meant to make definitive efficacy statements, data are presented as the mean ± 90% CI, and significance was reported at the P < .10 and P < .05 levels.8,25 On the basis of the strength values of patients at 26 weeks postoperatively,30 with a power of 0.80 and α of .05, we estimated that 17 patients per group would be necessary to detect a strength difference of 30% at 26 weeks postoperatively. Mixed-effects models determined the differences within either the placebo or the HGH group over time compared with preoperative values and also between groups at each time point. Values at baseline or for area under the curve (AUC) measurements between groups were compared using t tests. Fisher exact tests were used to compare the ACL graft type and adverse event risk. The Cohen d was calculated28 to determine the effect size of HGH treatment for chief parameters. Cohen d cutoffs were defined as very small (0.01), small (0.20), medium (0.50), large (0.80), very large (1.20), and huge (2.00).37 Analyses were performed using Prism (version 8.0; GraphPad Software).
RESULTS
Enrollment
We screened 273 male patients with a diagnosis of an ACL tear at our sports medicine center between October 2015 and October 2018 (Figure 1A). A total of 253 patients were excluded from the study, with 174 excluded for not meeting inclusion criteria either because they did not complete their rehabilitation at our center or because of a previous musculoskeletal injury of another joint. An additional 75 declined participation, with many citing intolerance of self-administered injections. There were 4 patients who were interested in the study and otherwise eligible but did not pass laboratory prescreening. A total of 20 patients were randomized to the placebo or HGH group. A participant in the placebo group withdrew after the first postoperative visit because of personal schedule changes, and that patient’s data are excluded from the results. Baseline patient characteristics are presented in Table 2.
TABLE 2.
Baseline Patient Characteristicsa
| Placebo (n = 9) | HGH (n = 10) | All (N = 19) | |
|---|---|---|---|
| Age, y | 24.6 ± 2.4 (20.3-33.4) | 28.2 ± 2.8 (18.1-34.0) | 26.5 ± 1.8 (18.1-34.0) |
| IGF-1, ng/mL | 269 ± 57 (155-397) | 230 ± 27 (173-303) | 249 ± 29 (155-397) |
| ACL graft type, n | |||
| Patellar tendon | 6 | 8 | 14 |
| Hamstring tendon | 3 | 2 | 5 |
Values are presented as mean ± 90% CI (range) unless otherwise indicated. Differences between the placebo and HGH groups were tested with the t test or Fisher exact test (ACL autograft type). No differences between groups were detected. ACL, anterior cruciate ligament; HGH, human growth hormone; IGF-1, insulin-like growth factor 1.
Safety
There were no significant differences in adverse events between the placebo and HGH groups (Table 3). A patient in the HGH group received a modified dose of HGH after reporting disruptive hyperhidrosis that he attributed to the study treatment. Although a causative relationship was not established, the dose for this patient was reduced by 50% in the final 2 weeks of the treatment window, and symptoms resolved. Despite reducing the total dose of HGH, this patient still had an IGF-1 AUC−1 to 5w that was 21% higher than the mean of the HGH group. Clinical blood work findings (Appendix Table A1, available in the online version of this article) were generally within acceptable normative values or expected to change as a result of surgery or rehabilitation interventions.
TABLE 3.
Adverse Eventsa
| Placebo | HGH | All | |
|---|---|---|---|
| Any adverse event | 7 (78) | 8 (80) | 15 (79) |
| Back pain | 4 (44) | 5 (50) | 9 (47) |
| Constipation | 0 (0) | 1 (10) | 1 (5) |
| Elevated alanine aminotransferase | 3 (33) | 2 (20) | 5 (26) |
| Elevated aspartate aminotransferase | 3 (33) | 2 (20) | 5 (26) |
| Elevated blood glucose | 1 (11) | 0 (0) | 1 (5) |
| Fatigue | 4 (44) | 4 (40) | 8 (42) |
| Headache | 3 (33) | 3 (30) | 6 (32) |
| Increased sweating | 2 (22) | 3 (30) | 5 (26) |
| Injection site bruising | 1 (11) | 0 (0) | 1 (5) |
| Insomnia | 0 (0) | 1 (10) | 1 (5) |
| Joint pain (other than surgical knee) | 2 (22) | 3 (30) | 5 (26) |
| Joint swelling (other than surgical knee) | 0 (0) | 1 (10) | 1 (5) |
| Muscle pain (other than surgical knee) | 1 (11) | 2 (20) | 3 (16) |
| Muscle spasms/cramps | 0 (0) | 1 (10) | 1 (5) |
| Reduced appetite | 0 (0) | 1 (10) | 1 (5) |
| Stiffness (other than surgical knee) | 0 (0) | 3 (30) | 3 (16) |
| Temperature sensation fluctuations | 0 (0) | 1 (10) | 1 (5) |
| Tingling sensation/numbness | 1 (11) | 2 (20) | 3 (16) |
Values are presented as n (%). Differences between the placebo and HGH groups were tested with the Fisher exact test. No significant differences between groups were observed. HGH, human growth hormone.
When reviewing the last data analysis report, the safety and data committee noted the 29% difference in relative isokinetic knee extension strength between the 2 treatment groups (discussed below), which was the primary outcome measure of the study. The results at this time point were statistically significant (P < .05) and had a large effect size (d = 0.80), and standard deviations were less in the current study than in the previous study used to perform the sample size estimates.30 Given the pilot nature of the study, and as the treatment assignments were unknown to the committee, the committee unanimously decided that it was not appropriate to continue the study given the substantial differences in strength between the groups. If the group that had received HGH was weaker, then the committee thought that it was inappropriate to continue to treat patients. Further, if the group that was stronger was receiving HGH, then there were sufficient data to design subsequent clinical trials. Therefore, the committee believed that the study had reached its original intended objectives.
Biomarkers
IGF-1 levels in both groups were the same at baseline, and all were within age-adjusted reference values. By 1 and 2 weeks postoperatively, IGF-1 levels in the HGH group were 2.4- and 2.8-fold greater than in the placebo group, respectively (Figure 2A). At 5 weeks, IGF-1 was 1.9-fold greater in the HGH group than in the placebo group, and from 12 weeks on, IGF-1 was not different between the groups (Figure 2A). Because of logistics, it was not possible to measure IGF-1 levels in every patient exactly when HGH was discontinued at 5 weeks, which likely contributed to the reduction of IGF-1 levels at the 5-week time point. For the overall treatment window, the AUC−1 to 5w for the HGH group was 2.1-fold higher than for the placebo group (Figure 2B), with a huge effect size (d = 2.93). We also evaluated myostatin, which is another circulating protein involved in the regulation of muscle atrophy. Healthy control participants had a myostatin level of 4.94 ± 0.40 ng/mL. Compared with the preoperative visit, there was a transient 33% reduction in myostatin in the placebo group and a 24% reduction in the HGH group, but levels returned to baseline, and no differences in AUC−1 to 5w for myostatin were observed (Figure 2, C and D).
Figure 2.
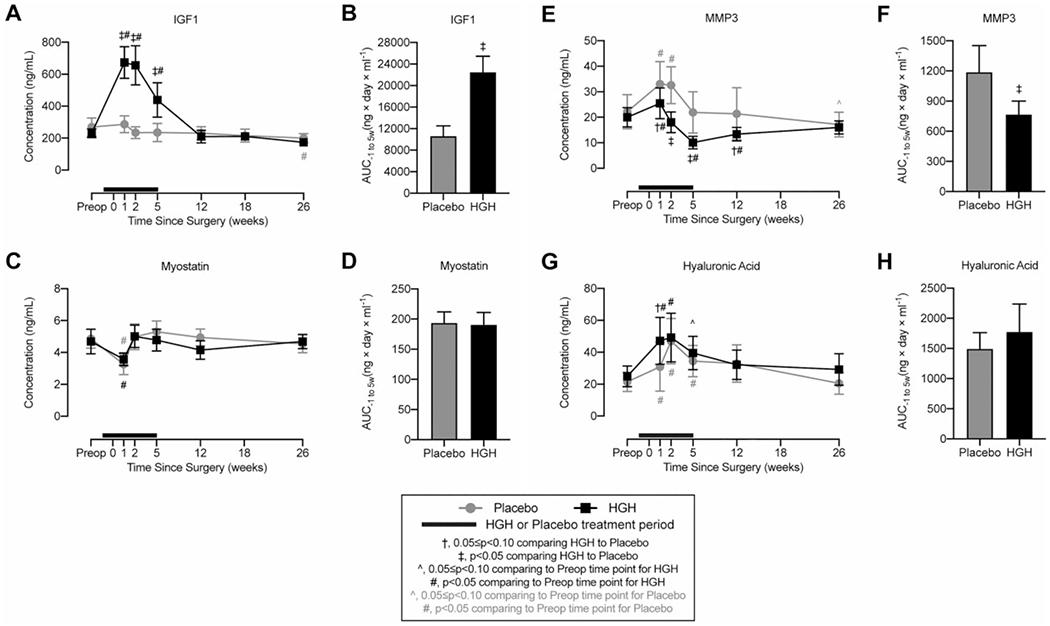
Circulating biomarkers. Changes in circulating concentration values from serum or plasma and the area under the curve (AUC) from 1 week before surgery to 5 weeks after surgery for (A, B) insulin-like growth factor 1 (IGF-1), (C, D) myostatin, (E, F) matrix metalloproteinase–3 (MMP3), and (G, H) hyaluronic acid (HA). Values are shown as mean ± 90% CI. Patients in the human growth hormone (HGH) group are shown in black, and those in the placebo group are shown in gray. The thick black line indicates the treatment window for HGH or placebo, with the preoperative time point occurring before initiating HGH or placebo treatment. Differences for A, C, E, and G were tested using mixed-effects models and for B, D, F, and H using t tests. Significance: †.05 ≤ P < .10 comparing HGH with placebo at a given time point; ‡P < .05 comparing HGH with placebo at a given time point; ^.05 ≤ P < .10 comparing the postoperative time point with the preoperative time point within the same treatment group; #P < .05 comparing the postoperative time point with the preoperative time point within the same treatment group.
For cartilage biomarkers, MMP3 was 22.9 ± 7.4 ng/mL in healthy control participants. MMP3 increased in both study groups at the first preoperative time point compared with baseline but was 23% lower in the HGH group (Figure 2E). By 2 weeks postoperatively, MMP3 was 45% lower in the HGH group, and at 5 and 12 weeks, MMP3 was 54% and 37% lower, respectively (Figure 2E). The AUC−1 to 5w for the HGH group was 36% lower than for the placebo group (Figure 2F), with a very large effect size (d = −1.34). HA of control participants was 18.6 ± 2.0 ng/mL. HA in the HGH group was 52% higher than in the placebo group at the first postoperative visit and was generally similar between the groups thereafter, with no difference in AUC−1 to 5w (Figure 2, G and H).
MRI Muscle Volume Measurements
The absolute quadriceps muscle volume in the HGH group was 17% higher than in the placebo group at baseline, but no differences were observed between the groups at 5 or 26 weeks (Figure 3A). Both groups lost approximately 20% of their absolute quadriceps muscle volume at 5 weeks after surgery, and by 26 weeks, the HGH group was 8% smaller than preoperative levels (Figure 3A). Similar results were observed for normalized quadriceps values (Figure 3B). For the hamstring muscle, the HGH group’s absolute volume was 14% larger than that of the placebo group (Figure 3C). Both groups lost approximately 10% of their volume at 5 weeks, and by 26 weeks, absolute volumes returned to preinjury levels (Figure 3C). The effect size for the significant difference between the placebo and HGH groups at 26 weeks was large (d = 0.91). Differences in normalized hamstring muscle volumes generally followed the same changes observed in absolute volumes, with a significant difference between the placebo and HGH groups at 26 weeks and a large effect size (d = 0.91) (Figure 3D).
Figure 3.
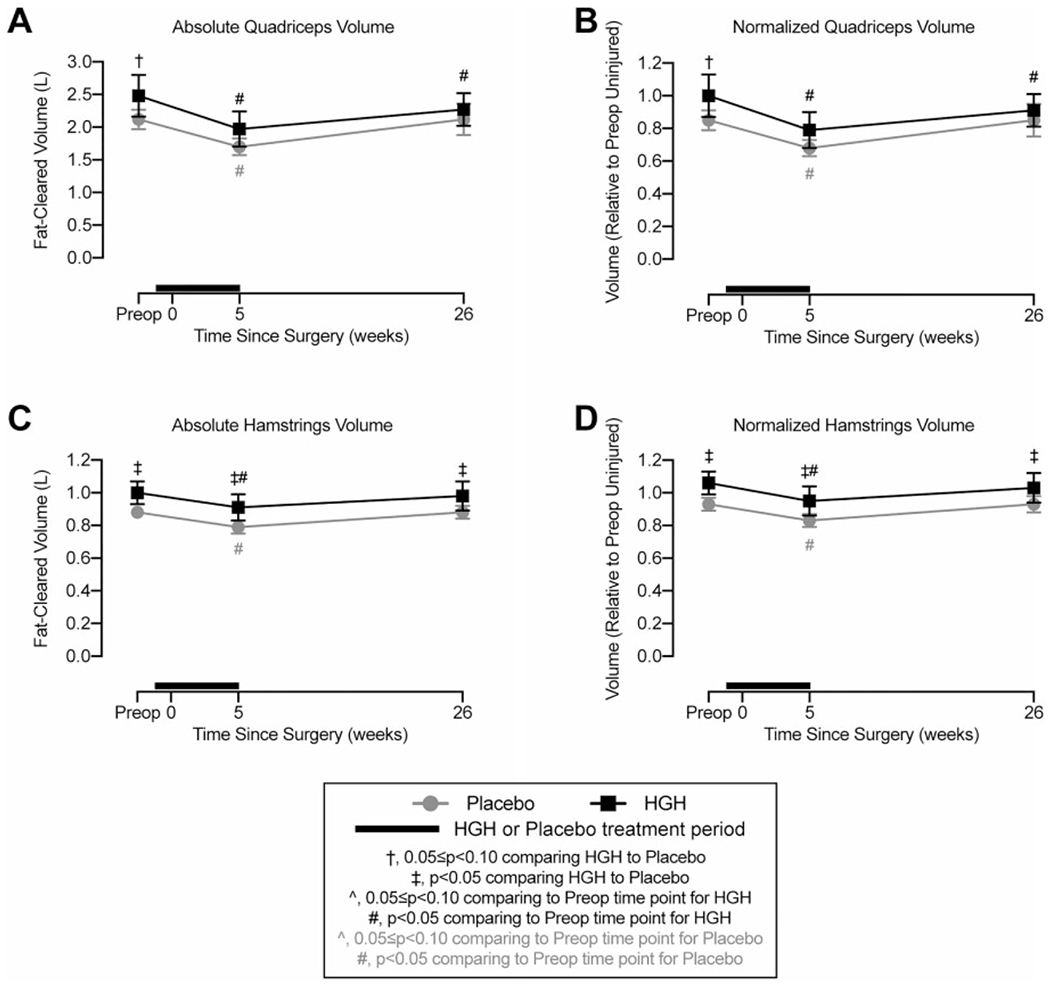
Magnetic resonance imaging volume measurements. Changes in fat-cleared (A) absolute and (B) normalized quadriceps muscle volume and (C) absolute and (D) normalized hamstring muscle volume. Values are shown as mean ± 90% CI. Patients in the human growth hormone (HGH) group are shown in black, and those in the placebo group are shown in gray. The thick black line indicates the treatment window for HGH or placebo, with the preoperative time point occurring before initiating HGH or placebo treatment. Differences were tested using mixed-effects models. Significance: †.05 ≤ P < .10 comparing HGH with placebo at a given time point; *P < .05 comparing HGH with placebo at a given time point; ^.05 ≤ P < .10 comparing the postoperative time point with the preoperative time point within the same treatment group; #P < .05 comparing the postoperative time point with the preoperative time point within the same treatment group.
Muscle Strength
For absolute isometric knee extension torque, both groups experienced a 59% reduction in strength from preoperatively to 5 weeks postoperatively, but by 26 weeks, the strength deficit of the placebo group was 18%, while the HGH group was not significantly different from preoperative values (Figure 4A). Normalized values showed similar trends but with smaller differences between the placebo and HGH groups (Figure 4B). There were no differences in absolute or relative isometric extension strength between the groups at any time point (Figure 4, A and B).
Figure 4.
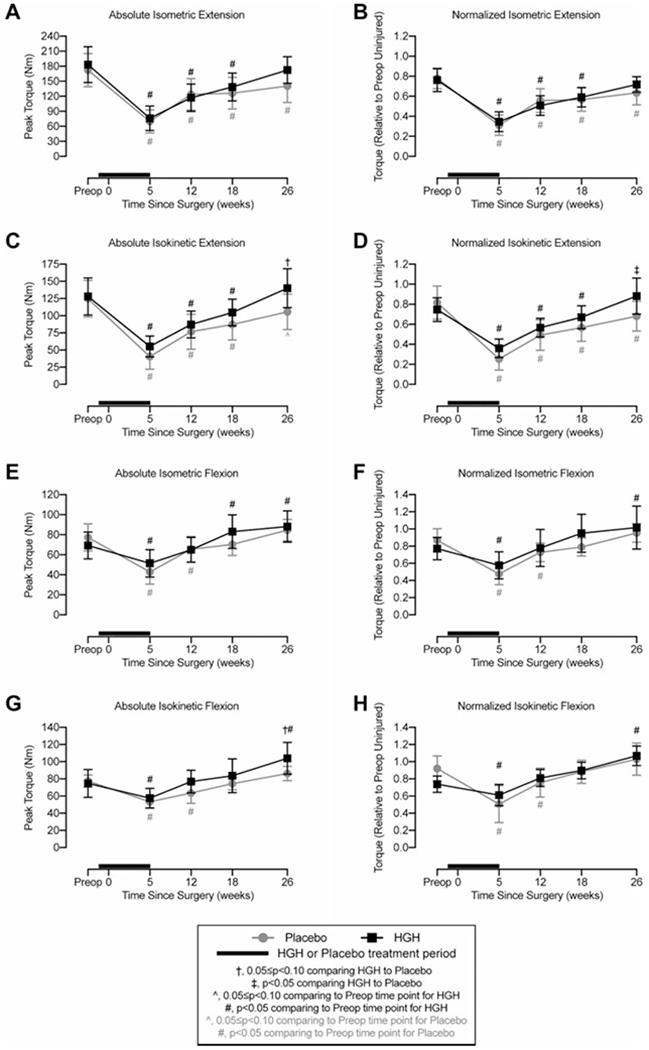
Strength measurements. Changes in peak (A) absolute and (B) normalized isometric knee extension at 90° of knee flexion, (C) absolute and (D) normalized isokinetic knee extension from 90° to 0° of knee flexion, (E) absolute and (F) normalized isometric knee flexion at 90° of knee flexion, and (G) absolute and (H) normalized isokinetic knee flexion from 0° to 90° of knee flexion. Values for B, D, F, and H are normalized to the muscle group in the contralateral uninjured leg before surgery. Values are shown as mean ± 90% CI. Patients in the human growth hormone (HGH) group are shown in black, and those in the placebo group are shown in gray. The thick black line indicates the treatment window for HGH or placebo, with the preoperative time point occurring before initiating HGH or placebo treatment. Differences were tested using mixed-effects models. Significance: †.05 ≤ P < .10 comparing HGH with placebo at a given time point; ‡P < .05 comparing HGH with placebo at a given time point; ^.05 ≤ P < .10 comparing the postoperative time point with the preoperative time point within the same treatment group; #P < .05 comparing the postoperative time point with the preoperative time point within the same treatment group.
Isokinetic strength measurements demonstrated a 68% loss in peak torque in the placebo group at 5 weeks, while the HGH group had a 58% reduction (Figure 4C). By 26 weeks, absolute torque was 15% lower in the placebo group than preoperative values, but the HGH group was 10% higher (Figure 4C) than preoperative values, resulting in a 33% increase in absolute torque in the HGH group compared with the placebo group. The effect size for this comparison was large (d = 0.80). Similar trends were present in normalized values, with the HGH group having a 29% increase in isokinetic torque compared with the placebo group and a large effect size (d = 0.80) (Figure 4D). Other than 26 weeks, no significant differences were observed between the groups (Figure 4, C and D).
For hamstring strength, the placebo group lost 44% of isometric knee flexion torque from preoperative values to 5 weeks after surgery, and the HGH group lost 25% (Figure 4E). At 26 weeks, the placebo group returned to preoperative values, while the HGH group was 27% higher than baseline values (Figure 4E). Normalized isometric flexion generally followed a similar pattern as absolute values (Figure 4F). There were no differences in absolute or relative isometric flexion strength between the groups at any time point (Figure 4, E and F).
Absolute isokinetic flexion values for both groups were reduced at 5 weeks, with a 32% and 23% loss in strength in the placebo and HGH groups, respectively. Also, 26 weeks after surgery, the placebo group returned to baseline, while the HGH group was 39% stronger than before surgery (Figure 4G). Between the groups, the HGH group had a 21% higher absolute torque than the placebo group at 26 weeks (Figure 4G), with a medium effect size (d = 0.75). For normalized values, at 26 weeks, the placebo group returned to preoperative values, while the HGH group was 32% stronger, and both groups returned to bilateral symmetry (Figure 4H). Other than absolute values at 26 weeks, no differences were observed between the groups for isokinetic flexion values (Figure 4, G and H).
Patient-Reported Outcome Scores
We obtained VR-12 (Figure 5, A and B), IKDC (Figure 5C), and KOOS (Figure 5, D–H) outcome scores from patients. For most scales associated with activity and function, both groups demonstrated a reduction at 1 and 2 weeks and generally returned to preoperative levels at 5 to 12 weeks after surgery. Other than a few instances, there were generally no differences between the placebo and HGH groups at any time points (Figure 5, A–G), with the exception of the KOOS symptoms subscale in which the HGH group was consistently lower than the placebo group (Figure 5H).
Figure 5.
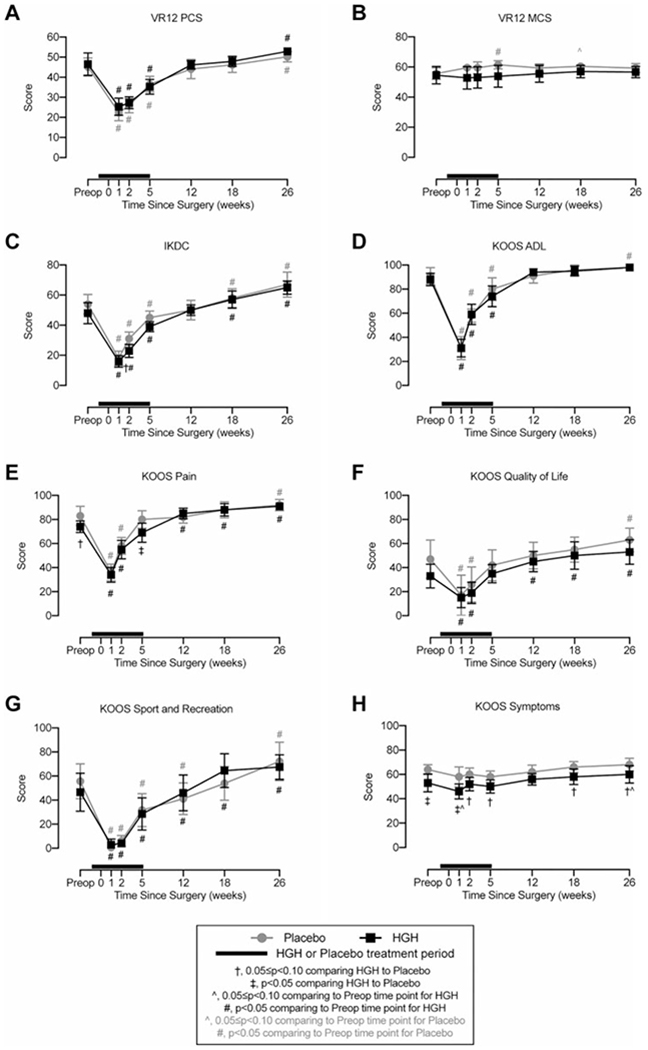
Patient-reported outcomes. Changes in patient-reported outcome scores. Higher scores correspond to improved functional status. (A) Veterans RAND 12-Item Health Survey (VR-12) physical component summary (PCS), (B) VR-12 mental component summary (MCS), (C) International Knee Documentation Committee (IKDC) form, and Knee injury and Osteoarthritis Outcome Score (KOOS) subscales for (D) activities of daily living (ADL), (E) pain, (F) quality of life, (G) sport and recreation function, and (H) other symptoms. Values are shown as mean ± 90% CI. Patients in the human growth hormone (HGH) group are shown in black, and those in the placebo group are shown in gray. The thick black line indicates the treatment window for HGH or placebo, with the preoperative time point occurring before initiating HGH or placebo treatment. Differences were tested using mixed-effects models. Significance: †.05 ≤ P < .10 comparing HGH with placebo at a given time point; ‡P < .05 comparing HGH with placebo at a given time point; ^.05 ≤ P < .10 comparing the postoperative time point with the preoperative time point within the same treatment group; #P < .05 comparing the postoperative time point with the preoperative time point within the same treatment group.
DISCUSSION
In the current double-blind, randomized controlled trial, HGH improved quadriceps strength and reduced MMP3 levels in patients undergoing ACLR. The primary outcome measure for this study was knee extension strength at 6 months. In support of our hypothesis, we observed a 33% increase in absolute torque and a 29% increase in relative torque in the HGH group compared with the placebo group at 6 months after ACLR. We are unaware of an established minimal clinically important difference for knee strength in patients undergoing ACLR, but the effect size was large. Despite the differences in force production, we did not observe similar responses in muscle size. However, changes in muscle fiber size and strength are not always linked.18 We previously evaluated changes in the size and force production of individual muscle fibers from patients undergoing ACLR, along with whole muscle isometric and isokinetic strength testing.15 This approach allowed us to eliminate contributions from the nervous system and directly measure force production at the cellular level. While we observed small reductions in muscle fiber size at 1 and 2 months after surgical repair, maximum isometric force production was nearly a third lower than in fibers from healthy controls and by 6 months was reduced by 27%.15 The trends in muscle fiber force closely matched changes in isokinetic and isometric torque values measured at the whole muscle level.15 Myofibrils are the organelles within muscle fibers that generate active tension, and these results indicated a reduction in functional myofibrils as a contributing factor to the weakness observed in patients after ACLR.15 Therapeutic interventions to increase myofibril abundance could therefore improve whole muscle strength and functional outcomes for patients undergoing ACLR.
Muscle fibers are multinucleated cells. The nuclei within existing fibers are terminally withdrawn from the cell cycle, and nuclei that undergo apoptosis can only be replaced by a local myogenic stem cell population, referred to as satellite cells.16 Immobilization can decrease muscle fiber nuclei and reduce muscle function,3 while resistance exercises induce satellite cell proliferation and fusion that then increase myonuclear abundance and force capacity.34 Patients with ACL tears have increased quadriceps myonuclear apoptosis and reduced satellite cell abundance,13,31 which suggest that muscle weakness in patients undergoing ACLR occurs at least in part because of defective satellite cell activity. IGF-1 induces satellite cell proliferation,16 and increased satellite cell activation is correlated with greater strength gains in resistance training programs,34 but the satellite cells of patients undergoing ACLR have deficits in activation after resistance exercises.12 IGF-1 may therefore protect patients undergoing ACLR from muscle weakness in part by promoting satellite cell activity, which could improve strength gains that result from resistance exercises in a postoperative rehabilitation program. Additionally, resistance exercises directly increase myofibrillar protein synthesis and force production in skeletal muscle. IGF-1 can activate the mTORC1 signal transduction pathway, which initiates protein synthesis in response to resistance exercises,16,19 and HGH could also have an additive effect on resistance exercise–induced increases in myofibrillar protein synthesis.
IGF-1 signaling can prevent muscle atrophy induced by the cytokine myostatin through blocking the pathways downstream of myostatin that direct proteolysis.16 Patients with ACL tears have increased myostatin protein abundance and markers of myostatin signaling in the quadriceps muscles of their injured limbs.32 Additionally, the targeted inhibition of myostatin was shown to protect against weakness in rats subjected to an ACL tear,43 which combined indicate a potential role for local myostatin signaling in causing muscle weakness after ACLR. In the current study, we did not observe an increase in systemic myostatin levels after ACLR, in disagreement with our previous findings.30 This is likely because of differences in the assay, as we previously used a competitive ELISA with polyclonal antibodies,30 while in the current study, we measured myostatin with a monoclonal antibody sandwich ELISA. There is a high degree of similarity between myostatin and other members of the transforming growth factor beta superfamily such as growth differentiation factor 11 (GDF11), and the cross-reactivity between myostatin and GDF11 has been documented.7 We have validated the ELISA in the current study using serum from mice in which the myostatin gene was genetically deleted,29 so we have confidence that myostatin levels measured in the current study are accurate. Although we did not detect an increase in circulating myostatin, previous studies that indicated a local elevation of myostatin in injured muscles,32 and the known ability of IGF-1 to inhibit the activation of signaling pathways that induce muscle weakness16 suggests that HGH may protect against the loss of strength in patients undergoing ACLR by inhibiting proteolysis.
Patients with ACL tears are at an increased risk of developing OA.21,35 IGF-1 promotes articular cartilage matrix protein synthesis and prevents cartilage catabolism11 and may therefore have a therapeutic role in preventing OA after ACLR. MMP3 is a stromelysin that is used as a marker of joint degradation.23 Circulating HA is used as a marker of synovial inflammation23 in OA, although it also has a chondroprotective role in joint health.6 We observed a consistent reduction in circulating MMP3 in the HGH group from postoperative weeks 1 through 12 and a transient increase in HA at the first postoperative visit. IGF-1 can downregulate MMP3 expression27 and increase HA synthase expression,24 which fit with the observed changes in circulating protein levels. These findings suggest a potential chondroprotective effect of HGH after ACLR that warrants a more robust evaluation.
There are several limitations in our study. We only included male patients, and while we think that the results are likely applicable to women, further studies should include both sexes. We only evaluated patients through 6 months after ACLR, and there could be continued improvements in strength beyond this point. We did not directly quantify changes in knee cartilage and articular morphology, which would provide greater insight into the effect of systemic HGH on the cartilage structure and the long-term development of OA. Local changes in histological or biochemical markers of muscle protein synthesis were not measured, although this could provide additional insight into the mechanism of action of HGH in the context of recovery from ACLR. There is muscle weakness that occurs immediately after an ACL tear before surgical reconstruction, and administering HGH closer to the time of the initial tear could further limit the loss in strength that occurs in these patients. We also did not measure electromyographic activity or perform hop tests or other functional agility tests. IGF-1 can reduce dorsal root ganglia nociceptive neuron sensitivity and modulate peripheral synaptic plasticity,44 and the therapeutic use of HGH could affect pain and neuromuscular function after ACLR. Enrollment was discontinued before reaching the preplanned number of 17 per group, which can result in overestimates of the effect in decisive trials,17 but we thought that this was the correct decision, as this was a pilot trial.
Fully restoring quadriceps muscle weakness remains an unmet clinical objective for most patients who undergo ACLR. The current pilot trial demonstrated that a 6-week perioperative course of HGH safely increased isokinetic quadriceps strength by nearly a third, although strength was not restored to level of the opposite limb. A meaningful effect of HGH on patient-reported outcome measures was not observed, but similar to previous studies, there was a disconnect between patient-reported function and objective measures of knee strength.15,30 While we cannot make definitive clinical recommendations about the use of HGH from the current study, additional trials that consider longer and perhaps higher doses of HGH in a larger population of male and female patients, along with neuromuscular and additional functional evaluations, would help to further determine the therapeutic potential ofHGH to improve outcomes for patients who undergo ACLR. HGH has the potential to cause negative systemic side effects and, like any drug, carries some risk with use. Aggregate data do not support an association between HGH and all-cause mortality, the development of primary neoplasms, hypertension, hyperlipidemia, or cerebrovascular events.1,39 However, HGH may increase the risk of developing type 2 diabetes mellitus in patients who are susceptible to this disease,1,39 which indicates the importance of carefully screening and monitoring insulin sensitivity in patients receiving HGH therapy. Finally, HGH is currently listed as a banned substance by the World Anti-Doping Agency,41 which limits the therapeutic use of HGH in high-level athletes. However, we think that HGH could potentially be used as part of a comprehensive ACLR rehabilitation plan and, perhaps for other traumatic joint injuries, to help athletes safely restore function and possibly protect against the development of OA without increasing strength or function to levels greater than they were before the injury.
TABLE 4.
Selected Anthropometric Data, Vital Signs, and Hematology and Clinical Chemistry Valuesa
| Preoperative | 1 wk | 2 wk | 5 wk | 12 wk | 18 wk | 26 wk | |
|---|---|---|---|---|---|---|---|
| Body mass index, kg/m2 | |||||||
| Placebo | 24.8 ± 1.5 | 26.2 ± 2.4 | 25.1 ± 1.6 | 24.6 ± 2.0 | 25.5 ± 1.9b | 25.3 ± 1.7b | 25.5 ± 1.5b |
| HGH | 26.5 ± 1.9 | 26.5 ± 2.2 | 26.4 ± 1.8 | 26.0 ± 1.7b | 26.5 ± 1.9 | 26.7 ± 1.9 | 26.6 ± 2.0 |
| Body surface area, m2 | |||||||
| Placebo | 1.96 ± 0.03 | 1.96 ± 0.07 | 1.95 ± 0.04 | 1.95 ± 0.05 | 1.98 ± 0.05b | 1.98 ± 0.04b | 1.98 ± 0.03b |
| HGH | 2.09 ± 0.10b | 2.10 ± 0.12b | 2.09 ± 0.10b | 2.08 ± 0.10b,c | 2.09 ± 0.10d | 2.10 ± 0.10b | 2.09 ± 0.11d |
| Resting heart rate, bpm | |||||||
| Placebo | 70.0 ± 7.0 | 68.0 ± 8.0 | 68.0 ± 4.0 | 69.0 ± 7.0 | 67.0 ± 4.0 | 68.0 ± 5.0 | 66.0 ± 4.0 |
| HGH | 61.0 ± 3.0b | 69.0 ± 7.0e | 72.0 ± 8.0b | 71.0 ± 6.0b | 66.0 ± 4.0 | 69.0 ± 5.0e | 63.0 ± 4.0 |
| Systolic blood pressure, mm Hg | |||||||
| Placebo | 121.0 ± 3.0 | 124.0 ± 3.0 | 123.0 ± 4.0 | 121.0 ± 3.0 | 120.0 ± 3.0 | 121.0 ± 2.0 | 121.0 ± 2.0 |
| HGH | 121.0 ± 3.0 | 124.0 ± 3.0 | 125.0 ± 4.0b | 123.0 ± 3.0 | 122.0 ± 4.0 | 125.0 ± 4.0b | 127.0 ± 3.0b,c |
| Diastolic blood pressure, mm Hg | |||||||
| Placebo | 75.0 ± 3.0 | 76.0 ± 2.0 | 76.0 ± 4.0 | 71.0 ± 2.0 | 72.0 ± 2.0e | 72.0 ± 3.0 | 73.0 ± 3.0 |
| HGH | 76.0 ± 3.0 | 77.0 ± 2.0 | 76.0 ± 4.0 | 77.0 ± 3.0c | 76.0 ± 3.0 | 79.0 ± 5.0c | 79.0 ± 5.0c |
| Hematocrit [40-50], % | |||||||
| Placebo | 45.3 ± 1.1 | 44.8 ± 2.3 | 42.6 ± 1.8b | 44.4 ± 1.4 | 44.5 ± 1.1 | 44.9 ± 1.1 | 44.4 ± 0.8 |
| HGH | 45.7 ± 1.2 | 45.2 ± 2.4 | 42.4 ± 2.0b | 44.1 ± 1.6e | 44.7 ± 1.3 | 44.5 ± 1.4 | 45.1 ± 1.4 |
| Hemoglobin [13.5-17.0], g/dL | |||||||
| Placebo | 15.5 ± 0.5 | 15.3 ± 0.8 | 14.6 ± 0.6b | 15.0 ± 0.5e | 15.2 ± 0.6 | 15.4 ± 0.5 | 15.2 ± 0.4 |
| HGH | 15.7 ± 0.4 | 15.5 ± 0.8 | 14.8 ± 0.6b | 15.1 ± 0.5b | 15.4 ± 0.4 | 15.4 ± 0.5 | 15.5 ± 0.4 |
| Red blood cells [4.4-5.7], M/μL | |||||||
| Placebo | 5.2 ± 0.1 | 5.2 ± 0.2 | 4.9 ± 0.2b | 5.1 ± 0.1 | 5.1 ± 0.1 | 5.2 ± 0.2 | 5.1 ± 0.1 |
| HGH | 5.3 ± 0.1 | 5.2 ± 0.3 | 5.0 ± 0.3b | 5.1 ± 0.2e | 5.2 ± 0.2 | 5.2 ± 0.2 | 5.2 ± 0.2 |
| White blood cells [4.0-10.0], K/μL | |||||||
| Placebo | 6.1 ± 0.7 | 8.0 ± 1.4b | 6.6 ± 0.9 | 6.2 ± 0.9 | 5.7 ± 0.8 | 6.1 ± 0.7 | 6.3 ± 1.5 |
| HGH | 6.1 ± 1.0 | 8.0 ± 1.0b | 6.6 ± 1.1 | 5.0 ± 0.5b,d | 5.5 ± 0.6 | 5.7 ± 0.9 | 5.4 ± 0.5 |
| Alanine aminotransferase [≤35], IU/L | |||||||
| Placebo | 23.6 ± 3.3 | 42.8 ± 14.2b | 24.4 ± 4.8 | 23.6 ± 8.3 | 30.6 ± 4.0 | 40.1 ± 18.2b | 25.1 ± 5.6 |
| HGH | 30.4 ± 5.5 | 41.4 ± 15.0e | 52.0 ± 22Ab,c | 42.6 ± 11.3c,e | 34.3 ± 10.5 | 26.3 ± 4.6d | 27.1 ± 4.3 |
| Alkaline phosphatase [30-130], IU/L | |||||||
| Placebo | 74.9 ± 13.1 | 84.3 ± 24.0e | 85.1 ± 17.2e | 75.3 ± 10.4 | 67.4 ± 10.0 | 69.8 ± 10.6 | 72.6 ± 12.6 |
| HGH | 71.7 ± 10.2 | 66.3 ± 10.6d | 63.4 ± 8.1c | 67.5 ± 8.1 | 76.1 ± 7.5 | 74.3 ± 8.8 | 73.4 ± 10.1 |
| Aspartate aminotransferase [8-30], IU/L | |||||||
| Placebo | 21.9 ± 2.5 | 31.9 ± 6.1 | 22.8 ± 1.8 | 21.2 ± 3.2 | 26.3 ± 3.3 | 87.6 ± 84.7b | 23.6 ± 3.1 |
| HGH | 31.0 ± 7.2 | 35.7 ± 7.8 | 34.8 ± 9.6 | 28.5 ± 5.2 | 28.6 ± 5.9 | 26.8 ± 3.3c | 27.9 ± 3.7 |
| Creatinine [0.7-1.3], mg/dL | |||||||
| Placebo | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1b | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1b |
| HGH | 1.0 ± 0.1d | 1.0 ± 0.1e | 1.0 ± 0.1b | 1.0 ± 0.1b,d | 1.0 ± 0.1e | 1.0 ± 0.1 | 1.0 ± 0.1e |
| Glucose [<130], mg/dL | |||||||
| Placebo | 92.7 ± 4.8 | 88.4 ± 4.1 | 87.1 ± 3.7 | 91.8 ± 6.3 | 91.0 ± 6.7 | 93.3 ± 11.1 | 86.7 ± 5.0 |
| HGH | 95.9 ± 4.6 | 94.9 ± 5.4 | 96.5 ± 3.6c | 95.0 ± 4.8 | 89.8 ± 4.6e | 91.3 ± 4.1 | 89.0 ± 4.1b |
| Hemoglobin A1c [4.2-5.6], % | |||||||
| Placebo | 5.2 ± 0.2 | 5.2 ± 0.2 | 5.2 ± 0.2 | 5.1 ± 0.2 | 5.1 ± 0.2 | 5.3 ± 0.1 | 5.1 ± 0.2 |
| HGH | 5.1 ± 0.1 | 5.1 ± 0.2 | 5.1 ± 0.2 | 5.0 ± 0.1b | 5.1 ± 0.1 | 5.1 ± 0.1 | 5.1 ± 0.2 |
| hs-CRP, mg/L | |||||||
| Placebo | 0.7 ± 0.3 | 11.5 ± 3.5b | 6.8 ± 3.2b | 1.2 ± 0.6 | 0.9 ± 0.5 | 1.3 ± 0.9 | 0.9 ± 0.6 |
| HGH | 1.9 ± 1.2 | 13.7 ± 4.5b | 5.5 ± 3.6b | 0.8 ± 0.6 | 1.3 ± 0.6 | 0.8 ± 0.4 | 1.2 ± 0.8 |
| HDL cholesterol [>40], mg/dL | |||||||
| Placebo | 59.4 ± 8.4 | 55.0 ± 10.4 | 53.9 ± 11.1 | 60.8 ± 11.7 | 58.9 ± 7.8 | 56.4 ± 10.1 | 57.9 ± 11.6 |
| HGH | 51.4 ± 8.1 | 42.8 ± 6.9 | 39.9 ± 4.8 | 46.8 ± 5.6d | 52.0 ± 5.3 | 65.0 ± 25.2b | 50.5 ± 7.0 |
| LDL cholesterol [<160], mg/dL | |||||||
| Placebo | 96.3 ± 19.1 | 100.9 ± 19.7 | 92.1 ± 15.4 | 87.8 ± 15.4 | 92.4 ± 15.1 | 93.9 ± 15.3 | 86.6 ± 14.0 |
| HGH | 104.6 ± 12.2 | 95.5 ± 12.5 | 86.5 ± 17.1e | 102.2 ± 10.9 | 110.0 ± 20.4 | 90.4 ± 21.8 | 103.6 ± 16.4 |
| Total cholesterol [<240], mg/dL | |||||||
| Placebo | 170.1 ± 23.7 | 175.9 ± 27.1 | 159.0 ± 18.4 | 165.3 ± 23.5 | 167.4 ± 18.3 | 165.3 ± 18.5 | 161.1 ± 18.3 |
| HGH | 176.0 ± 17.2 | 166.5 ± 14.5 | 158.4 ± 13.4b | 167.5 ± 16.7 | 180.0 ± 20.2 | 176.3 ± 21.9 | 172.3 ± 22.7 |
| Triglycerides [<200], mg/dL | |||||||
| Placebo | 72.0 ± 16.4 | 100.3 ± 18.5b | 64.6 ± 9.2 | 85.9 ± 22.3 | 80.3 ± 21.1 | 76.6 ± 18.2 | 82.6 ± 33.2 |
| HGH | 99.2 ± 28.5 | 126.3 ± 26.3b | 109.8 ± 36.3c | 93.1 ± 39.4 | 90.8 ± 15.8 | 87.0 ± 22.4 | 91.1 ± 34.6 |
Values are presented as mean ± 90% CI. Measures that have clinical normative values used by the study are shown in brackets. Differences were tested with a mixed-effects model. HDL, high-density lipoprotein; HGH, human growth hormone; hs-CRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein.
P < .05 comparing the postoperative time point to the preoperative time point within the same treatment group.
P < .05 comparing HGH with placebo at a given time point.
.05 ≤ P < .10 comparing HGH with placebo at a given time point.
.05 ≤ P < .10 comparing the postoperative time point to the preoperative time point within the same treatment group.
ACKNOWLEDGMENT
The authors acknowledge Ms Jaimee M. Gauthier, Mr Stuart M. Roche, Mr Dylan C. Sarver, Dr Christopher Robbins, Dr P. Troy Henning, Dr John Grant, Dr Bruce S. Miller, and Dr Edward M. Wojtys from the University of Michigan for their assistance in completing this study.
One or more of the authors has declared the following potential conflict of interest or source of funding: This study was supported with funding provided by the Mark Cuban Foundation. J.P.G. was supported by a fellowship from the National Institutes of Health (F31-AR065931). J.P.G. is currently employed by Merck. J.E.C. owns stock in Pfizer. T.M.A. has received general payments from Arthrex, Fujifilm SonoSite, and CDC Medical and research funding from Medical Device Business Services. J.A.J. has received consulting fees from Bioclinica and is on the advisory board for Philips. A.L.B. has received general payments from Genentech, Novartis, and Ipsen and research funding from Pfizer and Novartis. A.B. has received general payments from Arthrex, Smith & Nephew, Pivot Medical, Stryker, CDC Medical, and Flexion Therapeutics; research funding from Arthrex and GlaxoSmithKline; and royalties from Smith & Nephew. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
REFERENCES
- 1.Allen DB, Backeljauw P, Bidlingmaier M, et al. GH safety workshop position paper: a critical appraisal of recombinant human GH therapy in children and adults. Eur J Endocrinol. 2016;174(2):P1–P9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson AF, Irrgang JJ, Kocher MS, Mann BJ, Harrast JJ; Committee I. The International Knee Documentation Committee Subjective Knee Evaluation Form: normative data. Am J Sports Med. 2006; 34(1):128–135. [DOI] [PubMed] [Google Scholar]
- 3.Arentson-Lantz EJ, English KL, Paddon-Jones D, Fry CS. Fourteen days of bed rest induces a decline in satellite cell content and robust atrophy of skeletal muscle fibers in middle-aged adults. J Appl Physiol. 2016;120(8):965–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bidlingmaier M, Friedrich N, Emeny RT, et al. Reference intervals for insulin-like growth factor-1 (IGF-I) from birth to senescence: results from a multicenter study using a new automated chemiluminescence IGF-I immunoassay conforming to recent international recommendations. J Clin Endocrinol Metab. 2014;99(5):1712–1721. [DOI] [PubMed] [Google Scholar]
- 5.Boesen AP, Dideriksen K, Couppe C, et al. Tendon and skeletal muscle matrix gene expression and functional responses to immobilisation and rehabilitation in young males: effect of growth hormone administration. J Physiol (Lond). 2013;591(pt 23):6039–6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chevalier X, Jerosch J, Goupille P, et al. Single, intra-articular treatment with 6 ml hylan G-F 20 in patients with symptomatic primary osteoarthritis of the knee: a randomised, multicentre, double-blind, placebo controlled trial. Ann Rheum Dis. 2010;69(1):113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Egerman MA, Cadena SM, Gilbert JA, et al. GDF11 increases with age and inhibits skeletal muscle regeneration. Cell Metab. 2015;22(1): 164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eldridge SM, Lancaster GA, Campbell MJ, et al. Defining feasibility and pilot studies in preparation for randomised controlled trials: development of a conceptual framework. PLoS One. 2016;11(3): e0150205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esposito JG, Thomas SG, Kingdon L, Ezzat S. Anabolic growth hormone action improves submaximal measures of physical performance in patients with HIV-associated wasting. Am J Physiol Endocrinol Metab. 2005;289(3):e494–e503. [DOI] [PubMed] [Google Scholar]
- 10.Flosadottir V, Roos EM, Ageberg E. Muscle function is associated with future patient-reported outcomes in young adults with ACL injury. BMJ Open Sport Exerc Med. 2016;2(1):e000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fortier LA, Barker JU, Strauss EJ, McCarrel TM, Cole BJ. The role of growth factors in cartilage repair. Clin Orthop RelatRes. 2011;469(10): 2706–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedmann-Bette B, Profit F, Gwechenberger T, et al. Strength training effects on muscular regeneration after ACL reconstruction. Med Sci Sports Exerc. 2018;50(6):1152–1161. [DOI] [PubMed] [Google Scholar]
- 13.Fry CS, Johnson DL, Ireland ML, Noehren B. ACL injury reduces satellite cell abundance and promotes fibrogenic cell expansion within skeletal muscle. J Orthop Res. 2017;35(9):1876–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gullett NP, Hebbar G, Ziegler TR. Update on clinical trials of growth factors and anabolic steroids in cachexia and wasting. Am J Clin Nutr. 2010;91(4):1143S–1147S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gumucio JP, Sugg KB, Enselman ERS, et al. Anterior cruciate ligament tear induces a sustained loss of muscle fiber force production. Muscle Nerve. 2018;58(1):145–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gumucio JP, Sugg KB, Mendias CL. TGF-β superfamily signaling in muscle and tendon adaptation to resistance exercise. Exerc Sport Sci Rev. 2015;43(2):93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guyatt GH, Briel M, Glasziou P, Bassler D, Montori VM. Problems of stopping trials early. BMJ. 2012;344:e3863. [DOI] [PubMed] [Google Scholar]
- 18.Haun CT, Vann CG, Roberts BM, Vigotsky AD, Schoenfeld BJ, Roberts MD. A critical evaluation of the biological construct skeletal muscle hypertrophy: size matters but so does the measurement. Front Physiol. 2019;10:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heinemeier KM, Mackey AL, Doessing S, et al. GH/IGF-I axis and matrix adaptation of the musculotendinous tissue to exercise in humans. Scand J Med Sci Sports. 2012;22(4):e1–e7. [DOI] [PubMed] [Google Scholar]
- 20.Herzog MM, Marshall SW, Lund JL, Pate V, Mack CD, Spang JT. Trends in incidence of ACL reconstruction and concomitant procedures among commercially insured individuals in the United States, 2002–2014. Sports Health. 2018;10(6):523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ingersoll CD, Grindstaff TL, Pietrosimone BG, Hart JM. Neuromuscular consequences of anterior cruciate ligament injury. Clin Sports Med. 2008;27(3):383–404. [DOI] [PubMed] [Google Scholar]
- 22.Keays SL, Newcombe PA, Bullock-Saxton JE, Bullock MI, Keays AC. Factors involved in the development of osteoarthritis after anterior cruciate ligament surgery. Am J Sports Med. 2010;38(3):455–463. [DOI] [PubMed] [Google Scholar]
- 23.Kraus VB, Collins JE, Hargrove D, et al. Predictive validity of biochemical biomarkers in knee osteoarthritis: data from the FNIH OA Biomarkers Consortium. Ann Rheum Dis. 2017;76(1):186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuroda K, Utani A, Hamasaki Y, Shinkai H. Up-regulation of putative hyaluronan synthase mRNA by basic fibroblast growth factor and insulin-like growth factor-1 in human skin fibroblasts. J Dermatol Sci. 2001;26(2):156–160. [DOI] [PubMed] [Google Scholar]
- 25.Lee EC, Whitehead AL, Jacques RM, Julious SA. The statistical interpretation of pilot trials: should significance thresholds be reconsidered? BMC Med Res Methodol. 2014;14(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linge J, Borga M, West J, et al. Body composition profiling in the UK Biobank Imaging Study. Obesity (Silver Spring). 2018;26(11): 1785–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Z, Zhou K, Fu W, Zhang H. Insulin-like growth factor 1 activates PI3k/Akt signaling to antagonize lumbar disc degeneration. Cell Physiol Biochem. 2015;37(1):225–232. [DOI] [PubMed] [Google Scholar]
- 28.McGrath RE, Meyer GJ. When effect sizes disagree: the case of R and D. Psychol Methods. 2006;11(4):386–401. [DOI] [PubMed] [Google Scholar]
- 29.Mendias CL, Bakhurin KI, Gumucio JP, Shallal Ayzin MV, Davis CS, Faulkner JA. Haploinsufficiency of myostatin protects against aging-related declines in muscle function and enhances the longevity of mice. Aging Cell. 2015;14(4):704–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mendias CL, Lynch EB, Davis ME, et al. Changes in circulating biomarkers of muscle atrophy, inflammation, and cartilage turnover in patients undergoing anterior cruciate ligament reconstruction and rehabilitation. Am J Sports Med. 2013;41(8):1819–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noehren B, Andersen A, Hardy P, et al. Cellular and morphological alterations in the vastus lateralis muscle as the result of ACL injury and reconstruction. J Bone Joint Surg Am. 2016;98(18):1541–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peck BD, Brightwell CR, Johnson DL, Ireland ML, Noehren B, Fry CS. Anterior cruciate ligament tear promotes skeletal muscle myostatin expression, fibrogenic cell expansion, and a decline in muscle quality. Am J Sports Med. 2019;47(6):1385–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pietrosimone B, Pfeiffer SJ, Harkey MS, et al. Quadriceps weakness associates with greater T1p relaxation time in the medial femoral articular cartilage 6 months following anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2019;27(8): 2632–2642. [DOI] [PubMed] [Google Scholar]
- 34.Roberts MD, Haun CT, Mobley CB, et al. Physiological differences between low versus high skeletal muscle hypertrophic responders to resistance exercise training: current perspectives and future research directions. Front Physiol. 2018;9:834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roos EM. Joint injury causes knee osteoarthritis in young adults. Curr Opin Rheumatol. 2005;17(2):195–200. [DOI] [PubMed] [Google Scholar]
- 36.Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;1:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sawilowsky SS. New effect size rules of thumb. J Mod App Stat Meth. 2009;8(2):597–599. [Google Scholar]
- 38.Selim AJ, Rogers W, Fleishman JA, et al. Updated U.S. population standard for the Veterans RAND 12-item Health Survey (VR-12). Qual Life Res. 2009;18(1):43–52. [DOI] [PubMed] [Google Scholar]
- 39.Stochholm K, Kiess W. Long-term safety of growth hormone: a combined registry analysis. Clin Endocrinol (Oxf). 2018;88(4):515–528. [DOI] [PubMed] [Google Scholar]
- 40.Surya S, Horowitz JF, Goldenberg N, et al. The pattern of growth hormone delivery to peripheral tissues determines insulin-like growth factor-1 and lipolytic responses in obese subjects. J Clin Endocrinol Metab. 2009;94(8):2828–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.World Anti-Doping Agency. What is prohibited. https://www.wadaama.org/en/content/what-is-prohibited. Accessed July 14, 2019.
- 42.Wright RW, Haas AK, Anderson J, et al. Anterior cruciate ligament reconstruction rehabilitation: MOON guidelines. Sports Health. 2015; 7(3):239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wurtzel CN, Gumucio JP, Grekin JA, et al. Pharmacological inhibition of myostatin protects against skeletal muscle atrophy and weakness after anterior cruciate ligament tear. J Orthop Res. 2017;35(11): 2499–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu J, Casserly E, Yin Y, Cheng J. A systematic review of growth hormone in pain medicine: from rodents to humans. Pain Med. 2019;36(1):1. [DOI] [PubMed] [Google Scholar]


