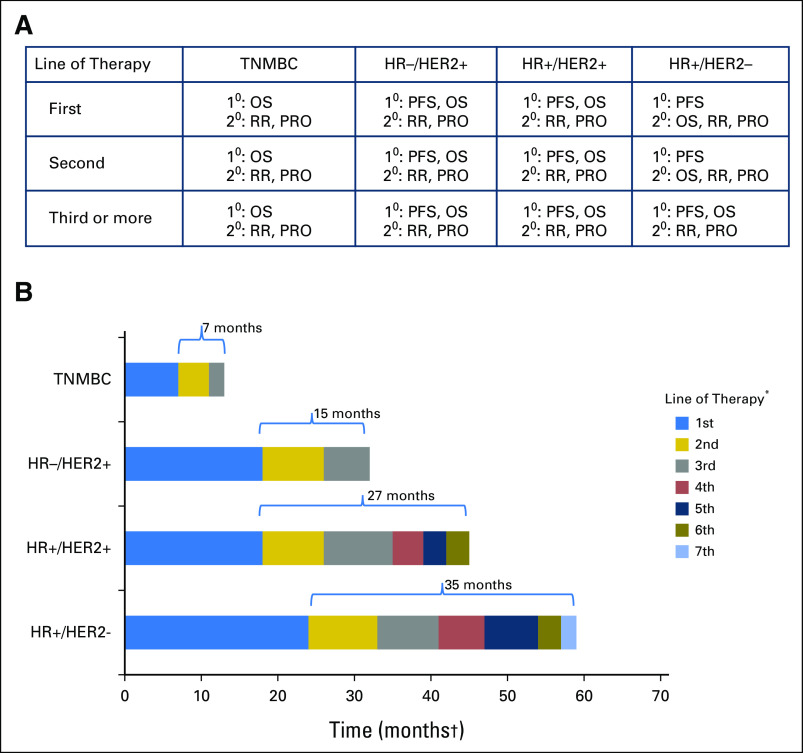
Fig 2.
(A) Working group consensus on preferred end points by biologic subtype and line of therapy. (B) Hypothetical scenarios for expected postprogression survival (PPS) and choice of preferred end point. In settings such as first-line treatment of triple-negative metastatic breast cancer (TNMBC) where expected PPS is < 12 months, overall survival (OS) is the preferred primary end point. In settings such as hormone receptor–negative (HR–)/human epidermal growth factor receptor 2–positive (HER2+) or HR+/HER2+ MBC where PPS is > 12 months, in both the first- and later-line settings, progression-free survival (PFS) is the end point of choice, and OS could be considered as a coprimary end point. In settings such as HR+/HER2– MBC, given the expected long PPS, PFS is the most appropriate end point. When such patients have disease that is refractory to endocrine therapy and have been exposed to several lines of chemotherapy, where PPS is expected to be much shorter, OS may be the most meaningful and appropriate end point. (*) Line of therapy may be endocrine therapy, chemotherapy, HER2-targeted therapy, combinations, and so forth. (†) Months shown are for illustrative purposes only. 1°, primary end point; 2°, secondary end point; PRO, patient-reported outcome; RR, response rate.