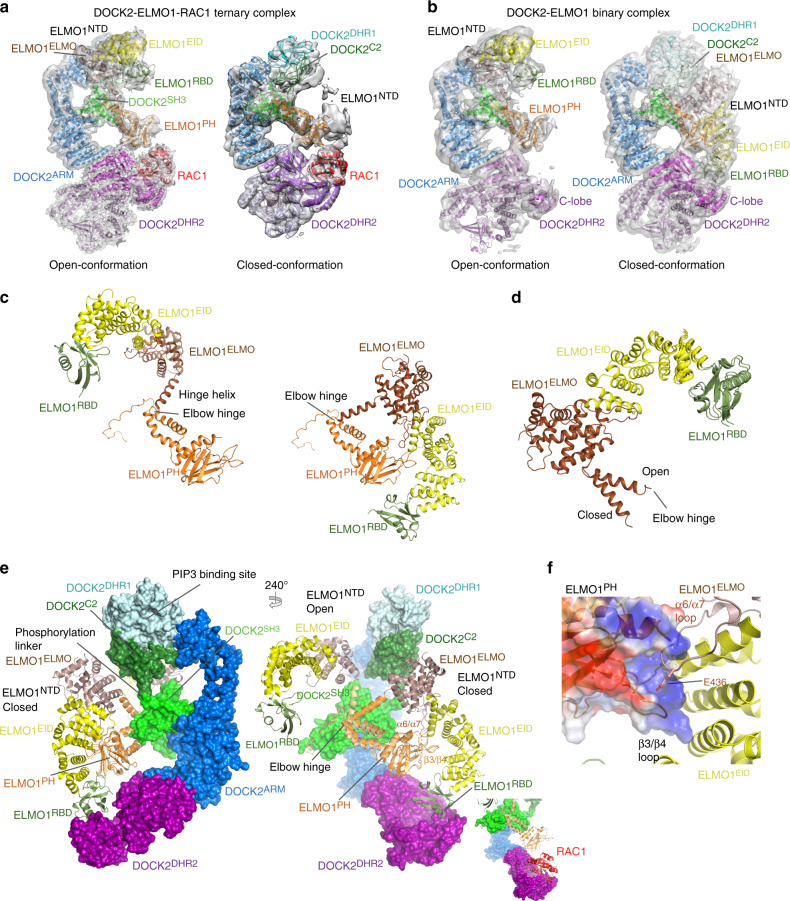
Fig. 3. Mechanism of DOCK2–ELMO1 activation.
a Comparison of the open- and closed-conformations of the DOCK2–ELMO1–RAC1 ternary complex. Left panel: cryo-EM density map of the complex adopting the open-conformation, with model coordinates placed into the map. Right panel shows the closed-conformation. ELMO1NTD is less well defined. b Comparison of the open- and closed-conformations of ELMO1 in the binary DOCK2–ELMO1 complex. A ribbon representation of the model has been placed in the cryo-EM density maps. c Ribbon representation of ELMO1 shown in the open-conformation of the ternary complex (left) and the closed-conformation of the binary complex (right). Domains are labelled and colour-coded according to Fig. 1a. d ELMO1NTD of the ternary and binary states are superimposed. This illustrates that the conformational change between the two states is confined to a rotation about the hinge elbow that connects ELMO1ELMO and ELMO1PH. For clarity ELMO1PH is omitted from Figure. e Two views showing superpositions of the open DOCK2–ELMO1–RAC1 ternary complex and the closed DOCK2–ELMO1 binary complex. In both views, DOCK2 of the ternary complex is shown as a surface representation, whereas ELMO1 is shown as a ribbon representation. This highlights the conformational rearrangement due to rotation of ELMO1 about the elbow hinge. In the closed-conformation of ELMO1, ELMO1RBD contacts DOCK2DHR2 at the RAC1-binding site, inhibiting interactions of RAC1 with DOCK2DHR2 (insert: ternary DOCK2–ELMO1–RAC1 complex). In the left view, the DOCK2 phosphorylation linker is labelled. In the closed-conformation of DOCK2–ELMO1, this region is in close proximity to ELMO1ELMO and the adjacent hinge helix, suggesting that phosphorylation at this site would favour the open active conformation. ELMO1PH of the binary and ternary complexes are coloured orange and light orange, respectively. f Details of the interface between ELMO1PH (displayed as an electrostatic potential surface) with ELMO1NTD, specifically the ELMO1ELMO and ELMO1EID domains of the DOCK2–ELMO1 binary complex.