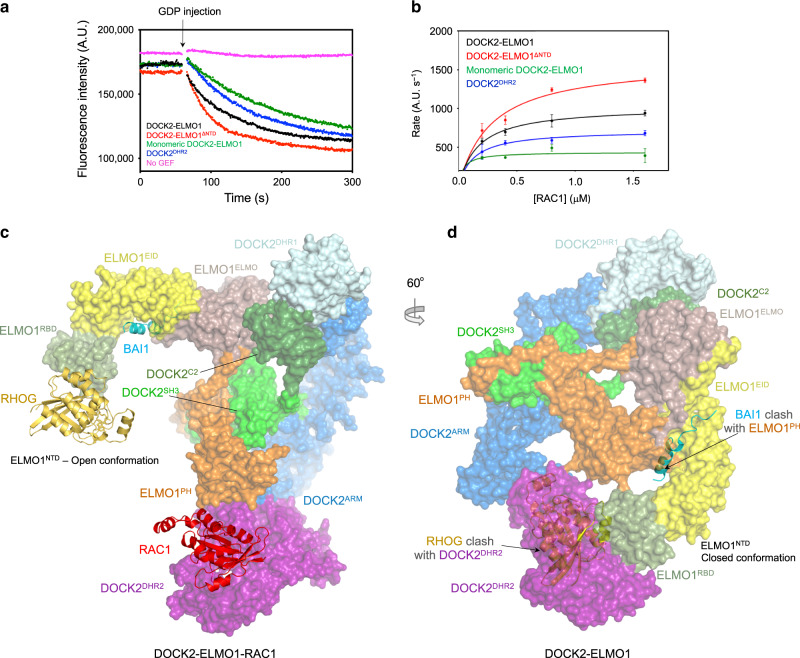
Fig. 6. ELMO1NTD auto-inhibits DOCK2–ELMO1.
a, b GEF activity data. ELMO1 stimulates DOCK2 GEF activity. Deleting ELMO1NTD stimulates DOCK2–ELMO1 GEF activity 60%. Disrupting the DOCK2 dimerization reduces GEF activity by ~60%. a Experimental data for RAC1 at 0.8 μM. There was no observable spontaneous GDP exchange without the GEF. GEF activity data was monitored by the exchange of fluorescent mant-GTP following an injection of GDP after 1 min (indicated by arrow). b Rates as a function of RAC1 concentration. The initial rates of exchange at increasing substrate (mant-RAC) concentrations were fitted to a Michaelis-Menten equation with the resultant constants shown in Table 1. Data are presented as mean values with error bars of +/− one standard deviation. The experiment shown in (a) and (b) was performed six times. c The crystal structure of the RHOG–ELMO2RBD complex was superimposed onto the modelled ELMO1RBD of DOCK2–ELMO1–RAC1 (shown in surface representation with ELMO1NTD in the open-conformation). d The crystal structure of the RHOG–ELMO2RBD complex was superimposed onto ELMO1RBD of DOCK2–ELMO1 (shown in surface representation with ELMO1NTD in closed-conformation). This shows that RHOG-binding site on ELMO1RBD is exposed in DOCK2–ELMO1–RAC1, whereas in the binary DOCK2–ELMO complex, with ELMO1 in the down conformation, the RHOG-binding site on ELMO1RBD is occluded by DOCK2DHR2. Modelling the ELMO2NTD–BAI1 complex onto ELMO1NTD of the DOCK2−ELMO1 binary complex (closed-conformation) shows that BAI1 bound to the ELMO1EID domain would clash with ELMO1PH (d), but not in the ternary DOCK2–ELMO1–RAC1 complex (c). Thus, RhoG and BAI1 would only bind to the activated conformation of the binary DOCK2−ELMO1 complex with the ELMO1NTD in the open-conformation with the DOCK2DHR2−RAC1 binding site exposed. Source data are provided as a Source Data file.