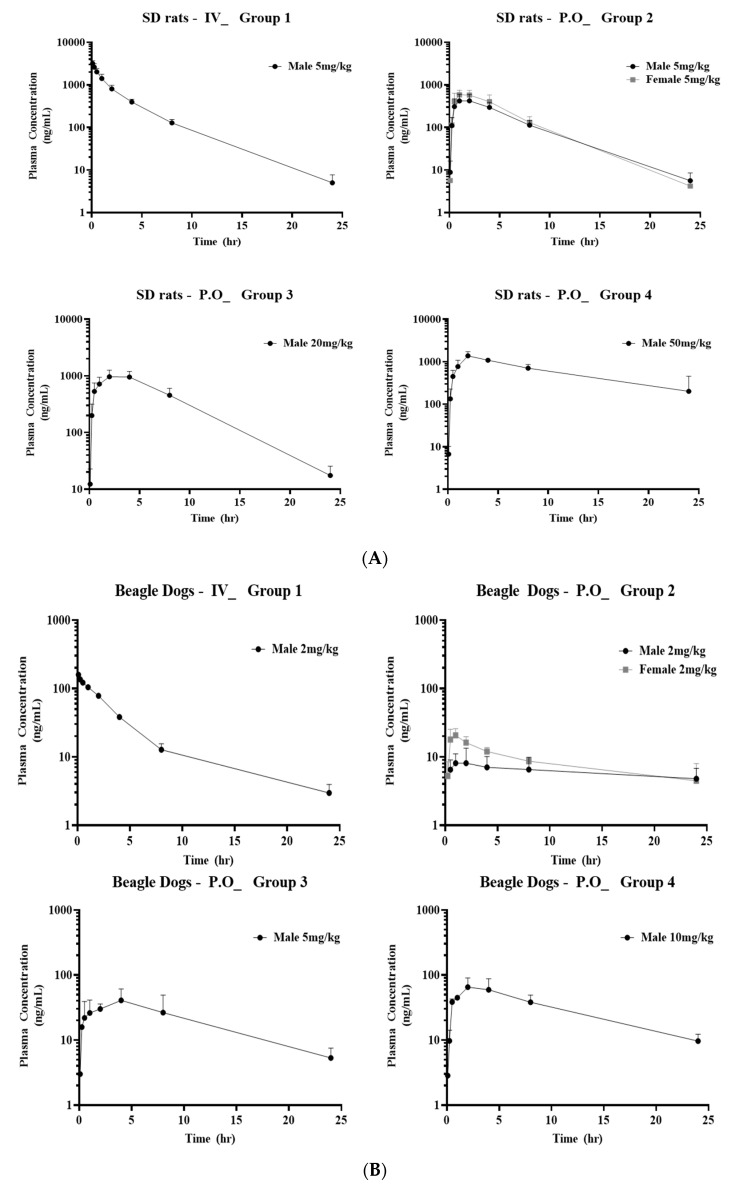
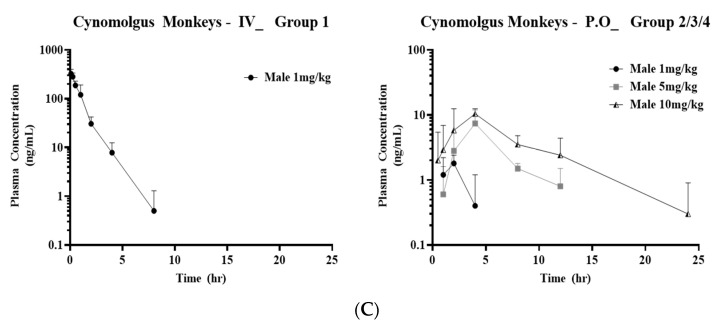
Figure 4.
Pharmacokinetics of ABN401. Plasma concentration–time profiles of ABN401 following intravenous and oral administration in (A) SD rats, (B) beagle dogs, and (C) cynomolgus monkeys. The pharmacokinetic profiles including F% (bioavailability), C0, Cmax, Tmax, T1/2, AUC0-t, and AUCinf were measured after intravenous (IV) administration at 5 mg/kg and oral (P.O) administration at 5, 20, or 50 mg/kg in SD rats and IV at 2 mg/kg and oral administration at 2, 5, and 10 mg/kg in beagle dogs. In the cynomolgus monkeys, systemic exposure to ABN401 was detected after a single oral administration at 1, 5, and 10 mg/kg and after a single IV dose of 1 mg/kg.