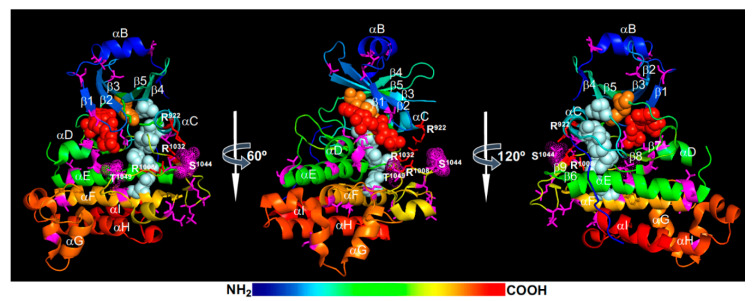
Figure 2.
A structural model of the activated BRI1 kinase domain. Shown here are three (0°, 60°, and 180°) rotational views of a rainbow-colored ribbon model of the crystal structure of the BRI1 kinase domain (Protein Data Bank No. 4oh4). Individual α-helices (αB-αI) and β-strands (β1-β5) were labelled. The magenta-colored sticks indicate phosphorylated Ser/Thr residues with the magenta dots surrounding Ser1044 and Thr1049 of the activation segment, the green sticks denote the Lys911 and Glu927 residues that form the salt bridge between the β3-strand and αC-helix, the red sticks mark the three positively charged residues of the phosphate-binding pocket, and the orange sticks represent the phosphorylated Tyr residue. The red spheres show adenylyl-imidodiphosphate (a non-hydrolysable ATP analog), the orange spheres indicate the gatekeeper Tyr956 residue that is also phosphorylated in in vitro assays, and the light-blue spheres denote the five regulatory-spine (R-spine) residues (from lower to upper: Asp1068, His1077, Phe1028, Ile931, and Leu942). The rainbow bar indicates the order of amino acids (AAs) from the N-terminus (blue) to the C-terminus (red).