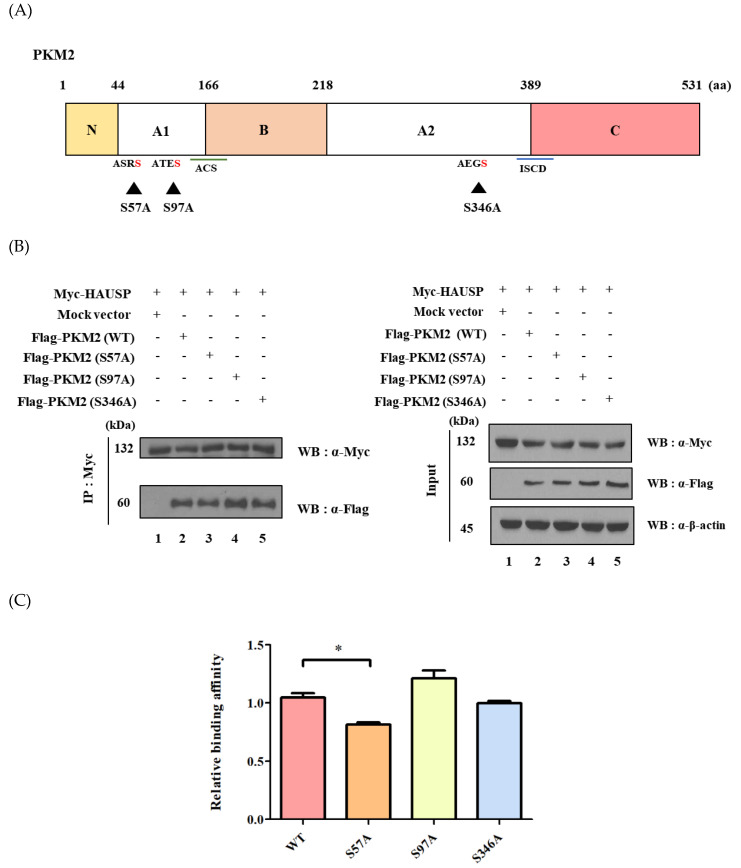
Figure 2.
Binding affinity between PKM2 mutants and HAUSP. (A) Schematic description of site-directed mutagenesis for PKM2. The catalytic active site (ACS) is located between the A1 and B domains of PKM2, and the intersubunit contact domain (ISCD) involved in the formation of tetrameric oligomers is located between the A2 and C domains. The C domain contains the allosteric activator (FBP) binding site and a nuclear localization signal sequence (NLS). N and C are the N-terminal and C-terminal domains, respectively [21]. The putative HAUSP binding motifs are ASRS, ATES, and AEGS. (B) HEK293T cells were both transfected with Myc-HAUSP and three different Flag-PKM2 mutant forms. Western blotting for HAUSP and mutant forms of PKM2 was performed. (C) Protein levels were determined using three separate experiments. (n = 3, * p < 0.05). Detailed information about western blotting can be found in Figure S2.