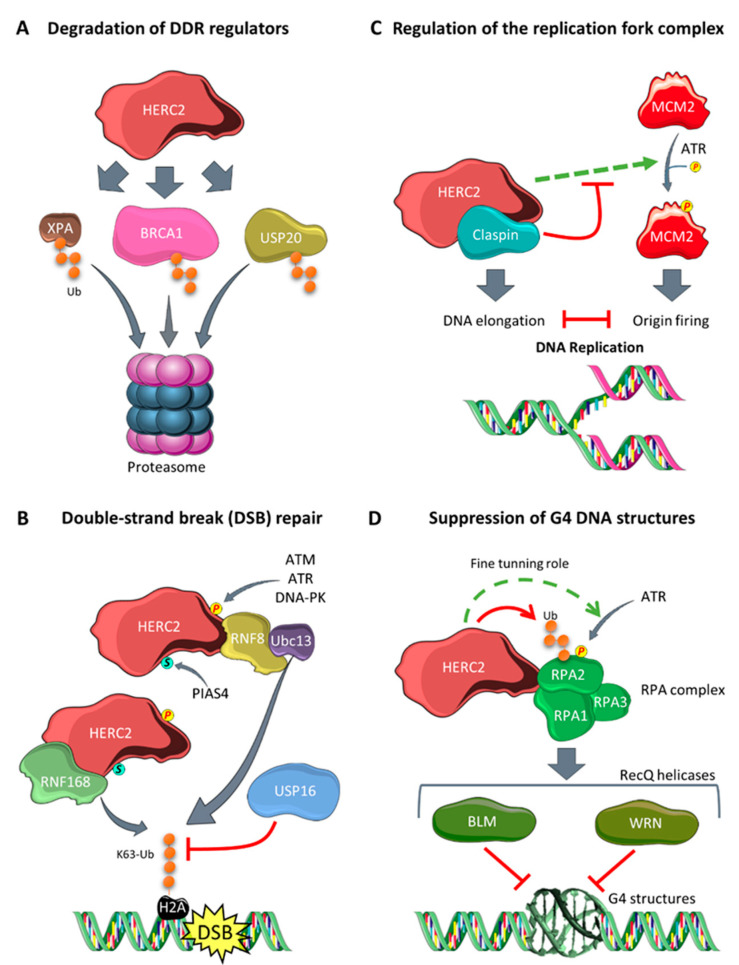
Figure 2.
Involvement of HERC2 in the regulation of genomic stability: (A) HERC2 catalyzes the polyubiquitylation of different DNA damage response (DDR) regulators, such as XPA, BRCA1, and USP20, targeting them for proteasomal degradation; (B) upon double-strand break in the DNA, HERC2 is phosphorylated (by ATM, ATR, or DNA-PK) and SUMOylated (by PIAS4). These posttranslational modifications allow HERC2 to bind to RNF8, promoting the specific assembly of RNF8 with the E2 enzyme Ubc13. This allows the formation of K63 polyubiquitin chains in H2A-type histones flanking the double-strand break site. HERC2 interacts with and stabilizes RNF168, and this amplifies the ubiquitin chain formation in histones. By contrast, USP16 levels increase in a HERC2-dependent manner and negatively regulate H2A histone ubiquitylation to fine-tune chain formation; (C) HERC2 is present in the replication fork complex that regulates the balance between DNA elongation and origin firing. HERC2 facilitates ATR-dependent MCM2 phosphorylation, which enhances origin firing, and this is inhibited by Claspin, another HERC2-interacting protein. (D) G4 structures cause replication stress, and this leads to HERC2 promoting RPA2 phosphorylation via ATR. Then, HERC2 polyubiquitylates the phosphorylated form of RPA2, targeting it for proteasomal degradation, and thereby fine-tuning the total levels of phospho-RPA2. This mechanism is essential for assembling the BLM and WRN RecQ helicases to the RPA complex and for its later role in suppressing G4 structures.