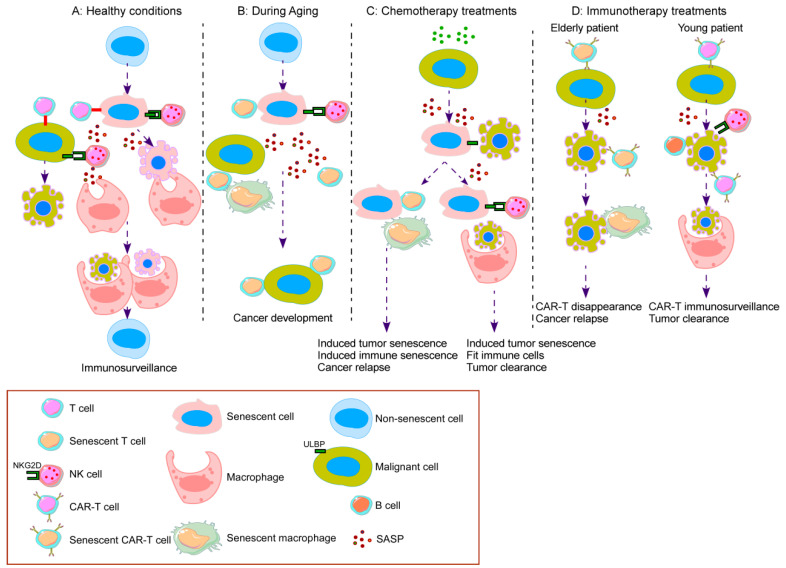
Figure 2.
The complex and contrasting roles of senescence in the immune response to cancer. (A) Healthy conditions: proliferative cells react to stresses, such as DNA damage or oncogene activation, by inducing senescence to avoid malignancy. Immune cells, recruited by immunosupportive elements of the SASP, prevent the harmful effects of senescent cell accumulation by mediating their clearance in a process known as immunosurveillance. As a further protective mechanism, malignant cells are detected and eliminated by effector T cells and NK cells. (B) During aging: immunosenescence leads to loss of immune cell function and thus a diminished ability to detect and clear senescent cells. The SASP released from persistent senescent cells has multiple roles in cancer progression, including promoting tumor cell expansion and the development of an immunosuppressive tumor microenvironment. Cancer cells reinforce T cell senescence through metabolite competition and other mechanisms. (C) Chemotherapy treatments: senescence-inducing chemotherapeutic agents force malignant cells into senescence. An undesirable effect of these chemotherapies is the induction of immune cell senescence, resulting in lack of senescent cell clearance and increased risk of cancer relapse. Nonetheless, when immune cell senescence is avoided, immune cells effectively remove senescent cancer cells and tumor progression is prevented. (D) Immunotherapy treatments: senescence in CAR-T cells causes cell cycle arrest and loss of function, and is therefore associated with decreased in vivo persistence and cancer relapse. This problem is likely to be more pronounced in elderly cancer patients due to immunosenescence of many immune cell types. Nondysfunctional chimeric antigen receptor modified T (CAR-T) cells expand in patients to become an effective antitumor therapy. Functional NK cells, macrophages and B cells also have important roles to play in tumor clearance, making these cell types exciting future immunotherapeutic options.