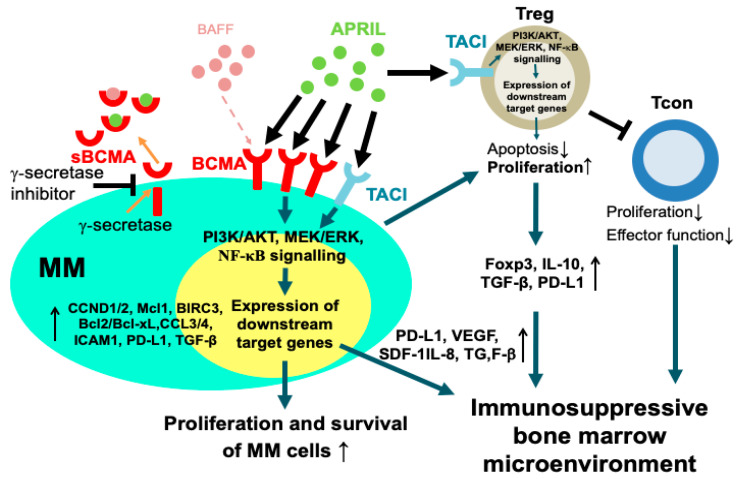
Figure 1.
The A-proliferation inducing ligand/B cell maturation antigen (APRIL/BCMA) pathway and its biological effect in the multiple myeloma bone marrow microenvironment. APRIL is mainly secreted from macrophages and osteoclasts in the bone marrow (BM) microenvironment. It binds to BCMA with significantly higher affinity than B-cell activation factor (BAFF) to critically regulate multiple myeloma (MM) cell growth, survival, and drug resistance. BCMA is expressed at significantly higher levels than transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI) in MM cells to constitutively transmit tumor-promoting signaling. In addition to upregulate key cell cycle progression (i.e., CCND1/2), anti-apoptotic proteins (i.e., Mcl1, Bcl-2, Bcl-xL, BIRC3), osteoclast-promoting factors (i.e., CCL3/4, SDF-1), and adhesion molecules (i.e., ICAM-1, CD44), APRIL/BCMA signaling cascade further induces major immunosuppressive factors (i.e., IL-10, PD-L1, TGF-β, VEGF) in MM cells. BCMA on the cell membrane of MM cells is shed by γ-secretase and soluble BCMA (sBCMA) is detected in serum samples of MM patients. Inhibitors blocking cleavage by γ-secretase can reduce the generation of sBCMA. MM cells further stimulate proliferation of regulatory T cells (Treg) via cell–cell contact and cytokine factor-dependent mechanisms. Despite no BCMA expression, regulatory T cells (Treg) utilizes TACI to deliver APRIL signaling pathway while conventional T cells (Tcon) rarely express TACI when compared with Treg. The interaction of APRIL with TACI induces the expression of proliferation and survival genes as well as central immunosuppressive markers (Foxp3, IL-10, PD-L1, TGF-β) in Treg but not Tcon. This signaling pathway enhances the inhibitory effects of Treg on conventional T cells (Tcon), thereby decreasing the proliferation and function of Tcon. Significantly, these signaling pathways contribute to the pathophysiology of MM cells and MM-induced immune suppression in the BM microenvironment.