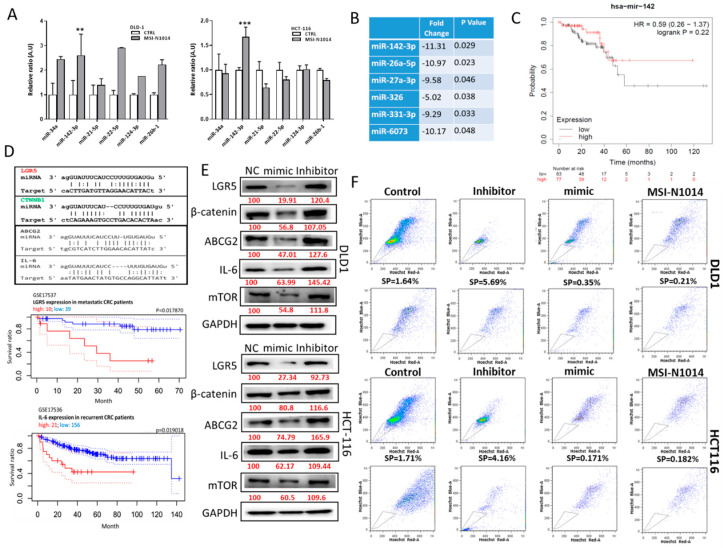
Figure 4.
MSI-N1014 treatment induced the tumor suppressor, miR-142-3p. (A) Small-scale microRNA (miR) screening for MSI-N1014-treated colorectal cancer (CRC) cells. Among different miRs tested, the miR-142-3p level had significantly increased in both HCT116 and DLD1 cells after MSI-N1014 treatment. (B) Tabulated plasma miR profiles between CRC and normal samples [13]. A significantly reduced level of miR-142-3p in plasma collected from patients with CRC (n = 61), compared to normal subjects (n = 24) was identified. (C) Kaplan-Meier survival curve of a TCGA cohort suggested that a higher miR-142 was positively correlated (p = 0.22) with a higher survival ratio in patients with CRC. (D) Table listing potential targets of miR-142-3p. Leucine-rich repeat-containing G-protein coupled receptor 5 (LGR5) was predicted and experimentally validated as a strong target for miR-142-3p, while CTNNB1 (β-catenin), interleukin (IL)-6, and ATP-binding cassette super-family G member 2 (ABCG2) were also predicated targets for miR-142-3p. (E) Western blot analysis validating targets of miR-142-3p. Both DLD1 and HCT116 cells were transfected with miR-142-3p-mimic and inhibitor molecules. Mimic transfected samples showed markedly reduced expressions of LGR5, β-catenin, ABCG2, mammalian target of rapamycin (mTOR), and IL-6, while the opposite occurred in inhibitor-transfected samples. (F) Side-population (SP) flow cytometric assay. Percentages of SP cells were prominently reduced in miR-142-3p-transfected DLD1 and HCT116 cells, while they were increased in the inhibitor-transfected counterparts. Numbers in red indicate the relative expression ratio. ** p < 0.01, *** p < 0.001.