Table 1.
Main characteristics of major PARPi.
| Drug | Pharmaceutical Company | Chemical Formula | 2D Structure | Catalytic Site | EMA Registration |
|---|---|---|---|---|---|
| Olaparib/AZD-2281 | AstraZeneca | C24H23FN4O3 |
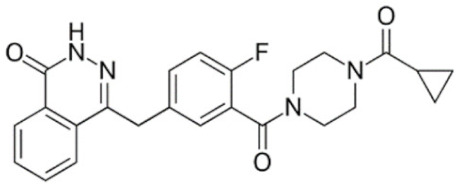
|
PARP 1, 2 and 3 | December 2014 |
| Niraparib/MK-4827 | Tesaro/GSK | C19H20N4O |
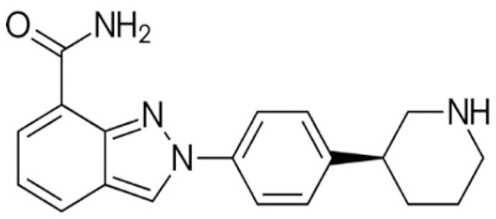
|
PARP 1 and 2 | November 2017 |
| Rucaparib/AG-014699 | Clovis oncology | C19H18FN3O |
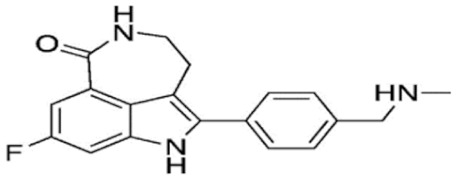
|
PARP 1, 2 and 3 | May 2018 |
| Talazoparib/BMN-673 | Pfizer | C19H14F2N6O |
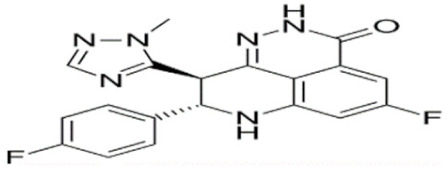
|
PARP 1 and 2 Powerful PARP trapping |
June 2019 |
| Veliparib/ABT-888 | AbbVie | C13H16N4O |
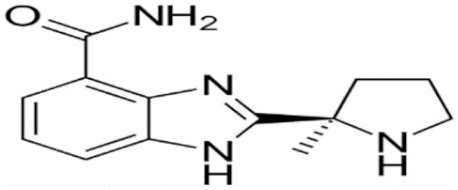
|
PARP 1 and 2 Weakest PARP trapping |
Phase III |
| Pamiparib/AG-14361 | BeiGene | C19H20N4O |
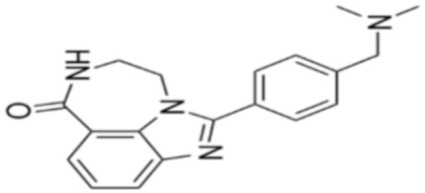
|
PARP 1 | Phase II |
PARPi—PARP inhibitors; EMA—European medicines agency; PARP—poly ADP ribose polymerase.
