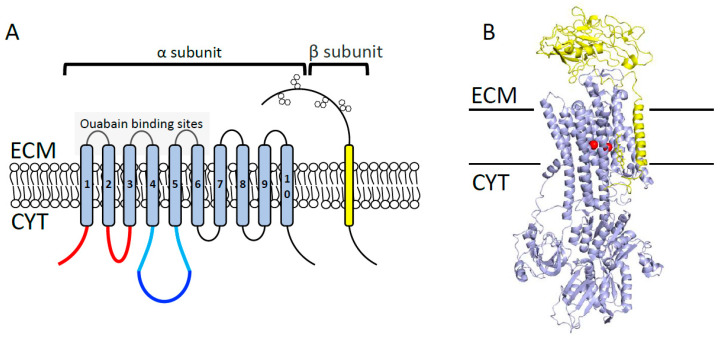
Figure 2.
Na+-, K+-ATPase overall structure. (A) Na+-, K+-ATPase consists of a catalytic α subunit and a regulatory β subunit. The α subunit consists of 10 transmembrane helices, harboring 3 different cytoplasmic domains: the actuator responsible for dephosphorylation (shown in red); the nucleotide-binding, responsible for ATP binding (shown in blue); and the phosphorylation domains (shown in cyan). The β subunit consists of one transmembrane helix with a large glycosylated extracellular domain (shown in hexagon orange boxes). ECM = extracellular milieu; CYT = cytoplasm. (B) Overall domain architecture of Na+/K+ transporter in the Na+-bound state (Protein Data Bank [PDB] code 4HQJ). Catalytic α subunit is colored in blue, β subunit is shown in yellow, and Na+ ions are shown in red.