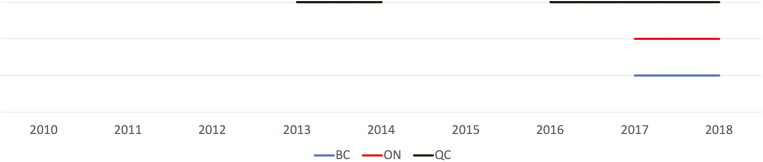
Figure 1.
Time-varying policy changes by province. The solid lines represent calendar time when no fibrosis stage restrictions were in place. No line represents that either significant (>F2) or advanced (>F3) liver fibrosis stage restrictions were required for reimbursement of direct-acting antivirals (DAAs). Before 2013, due to the lower efficacy and higher toxicity of interferon-based therapies in human immune deficiency virus (HIV)-hepatitis C virus (HCV) coinfection, only a very few patients—typically, only people with advanced fibrosis and at increased risk for short-term adverse outcomes—were treated with these agents. In Quebec, in 2014, simeprevir and sofosbuvir were unrestricted for patients living with HCV. Although HIV infection was a listed restriction, coinfected patients were usually granted access on a case-by-case basis through the “patient d’exception” process. As of 2016, people coinfected with HIV and HCV were considered a priority population and sofosbuvir/ledipasvir and ombitasvir/paritaprevir/ritonavir dasabuvir were available without fibrosis stage restrictions; sofosbuvir/velpatasvir was available without restrictions from 2017. In British Columbia and Ontario, in 2017, after the pan-Canadian Pharmaceutical Alliance used collective bargaining to reduce DAA drug prices across Canada, provinces removed fibrosis stage restrictions as a criterion for treatment reimbursement. No sobriety restrictions were present in Canada. Abbreviations: BC, British Columbia; ON, Ontario; QC, Quebec.