Fig. 1.
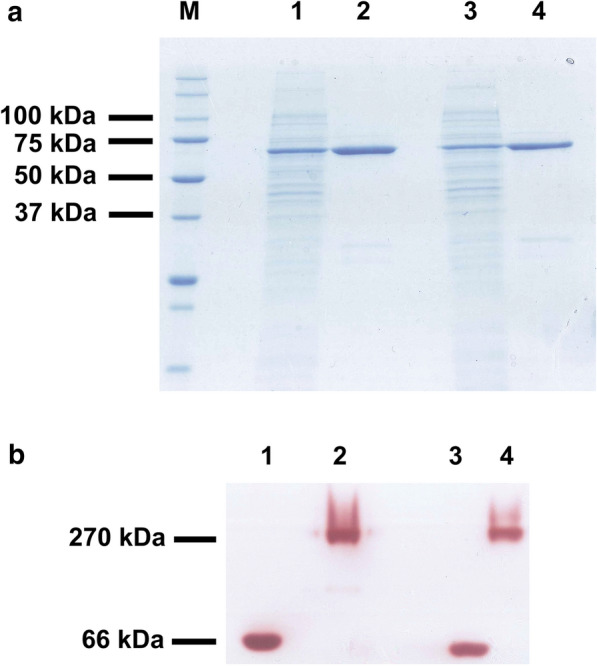
SDS-PAGE analysis of the α-glucosidases from Metsnikowia species expressed in E. coli. Non-precipitated cell lysates of the bacteria including the constructions Mg-αGLU-pET28b(+) (lane 1) and Mr-αGLU-pET28b(+) (lane 3) were loaded. Ni-Sepharose purified fractions containing recombinant proteins Mg-αGlu (lane 2) and Mr-αGlu (lane 4) were also evaluated (a). Zymogram analysis using 1 M sucrose as substrate and purified Mg-αGlu (lane 1) or Mr-αGlu (lane 3). Commercial S. cerevisiae invertase was used as positive control (lanes 2 and 4) (b). Proteins in the range of 1–3 μg were loaded in each well. Numbers at the left of panels (a) and (b) indicate the positions of the molecular mass standards in kDa
