Abstract
Background
Multidrug resistance in Acinetobacter baumannii is constantly on the rise. There has also been an increase in the morbidity and mortality of patients with infection by the same pathogen.
Aim
This study aimed to assess the patterns of antibiotic resistance exhibited by various clinical isolates of Acinetobacter baumannii, examine the risk factors associated, and investigate the prevalence of co-infecting pathogens and the clinical outcomes of the patients.
Study Design
Retrospective cross-sectional study.
Patients and Methods
Reports of 100 isolates of Acinetobacter baumannii obtained from patients admitted in two tertiary hospitals were used for the study. Identification and determination of antibiotic resistance patterns were done using Vitek2. The presence of probable risk factors was noted. The pattern of clinical outcomes of the patients and the prevalence of co-infecting pathogens were analyzed. Data analysis was done using descriptive statistics.
Results
More than 50% of isolates showed resistance independently to imipenem and meropenem. Higher rates of susceptibility were observed with tigecycline (55%). Isolates obtained from patients in the intensive care unit (ICU) showed resistance to a more number of antibiotics than those in the wards and operation theatre. Seventeen percent of the isolates were associated with a co-infecting pathogen such as Pseudomonas, Enterococcus, Klebsiella, 87% of the patients were discharged, 12% expired, and 1% were shifted. A positive correlation was found between the duration of hospital stay and number of antibiotics to which the isolate was resistant.
Conclusion
Multidrug resistance in Acinetobacter baumannii continues to be a menace. In this study, a large number of isolates exhibited resistance to carbapenems such as imipenem, meropenem, and ertapenem, thereby signifying the need for further research and the use of other antibiotics such as tigecycline, to which higher susceptibility was observed.
Keywords: multidrug resistance, clinical outcomes, nosocomial infection
Introduction
Medicine, in the modern world, is a highly active branch of science with advancements happening at remarkable rates. Despite several discoveries and the advent of technology, antibiotic resistance continues to remain a significant source of concern in the field of therapeutics. It has been attributed to the overuse and misuse of antibiotics,1 thereby decreasing the efficacy of these marvelous drugs, once considered “magic bullets” by the great Paul Ehrlich.2 Insufficient attention to a problem as disturbing as this, could lead to disastrous consequences and hinder the progress of therapeutics. Amongst various organisms exhibiting antibiotic resistance, Acinetobacter baumannii has, of late, emerged as an exceedingly troublesome pathogen. Due to its ability to display a wide variety of antibiotic resistance determinants, and to undergo frequent modifications in the same, it is currently a matter of considerable significance to the medical community.3 It is a gram-negative aerobic coccobacillus.4 It is nonfastidious, non-motile, does not ferment sugars, is negative for oxidase and positive for catalase.5 It is found ubiquitously in the environment.6 It contains proteins such as porins and efflux channels, which play a significant role in resistance mechanisms.7 It is a common cause of hospital-acquired infections and has been known to increase the duration of hospital stay.8 It is associated with several diseases such as pneumonia, urinary tract infection, skin and wound infection, bacteremia, meningitis, and endocarditis.9 The two significant factors governing the success of Acinetobacter baumannii are its ability to adapt to the hospital environment, and antibiotic resistance.10 Though it is prevalent as a hospital-acquired infection, there have been occasional cases of patients presenting with community-acquired colonization of wounds.11 They may also colonize invasive devices such as tracheostomy tubes, endotracheal tubes, abdominal catheters, Foley’s catheters, and other devices used for monitoring of fluids, providing medication, or life-saving support.12 This retrospective study aimed to investigate the resistance patterns of obtained isolates, associated risk factors, co-infecting pathogens, and clinical outcomes of patients with infection. It is necessary to study and understand the characteristics of patients who are colonized or infected with multidrug-resistant Acinetobacter so that treatment with appropriate antibiotics may be initiated without delay.13 This practice would help in decreasing the acquisition of newer resistance mechanisms by early destruction of the organisms, thereby reducing the prevalence of infections by the same. This was a retrospective study conducted using hospital database of multidrug-resistant Acinetobacter isolates, obtained from patients admitted in two tertiary hospitals in Mangalore. The objectives of this study were to study the patterns and levels of antibiotic resistance exhibited by various clinical isolates of Acinetobacter baumannii, to examine the risk factors associated with infection in patients, to investigate the prevalence of co-infecting pathogens and the clinical outcomes in patients with the infection.
Patients and Methods
The proposed study was conducted in a microbiology diagnostic laboratory located in Mangalore, India. The study proposal was submitted to the Institutional Ethics Committee, Kasturba Medical College, Mangaluru (Reg. No. ECR/541/Inst/KA/2014/RR-17), and the same was approved. In compliance with the Declaration of Helsinki, IRB granted the patient consent waiver to review their medical record in this retrospective study where the participants could not be contacted, and there was absolutely no risk to the patient, all personally identifiable information was removed from the datasheet (anonymization). It was an efficient way of gaining a comprehensive view of the health system’s response to a particular medical problem as an effective tool for scientific study. The study was performed for two months. This study was a retrospective review of the hospital database of two tertiary hospitals in Mangalore and included 100 multidrug-resistant Acinetobacter isolates. There were no patients with multiple strains. Identification and estimation of the minimum inhibitory concentration of all clinical isolates of Acinetobacter baumannii were done using the Vitek2 system.14 Multidrug-resistant Acinetobacter was defined as strains which showed resistance to greater than or equal to three classes of drugs including carbapenems.15 Demographic details such as age, sex, the location of stay were obtained from the database. Further, the presence of probable risk factors such as underlying comorbidity, prolonged hospital stay, diabetes mellitus, invasive devices procedures such as tracheostomy, abdominal drain, and several others, were noted.16 Antibiogram recordings were checked to analyze the resistance patterns in the isolates obtained. Data analysis was done by descriptive statistics, using SPSS 17.
Results
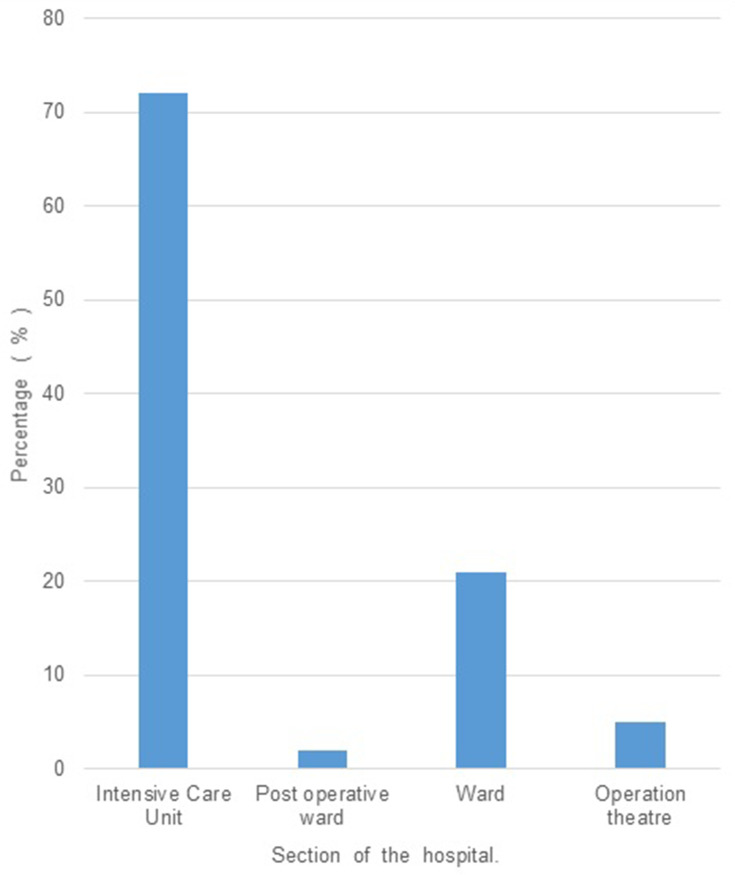
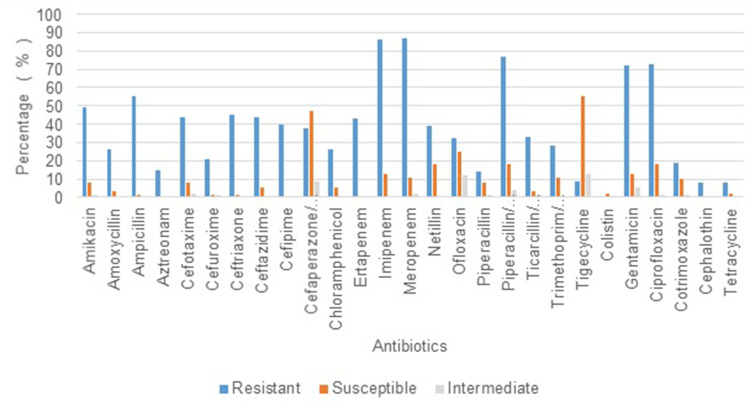
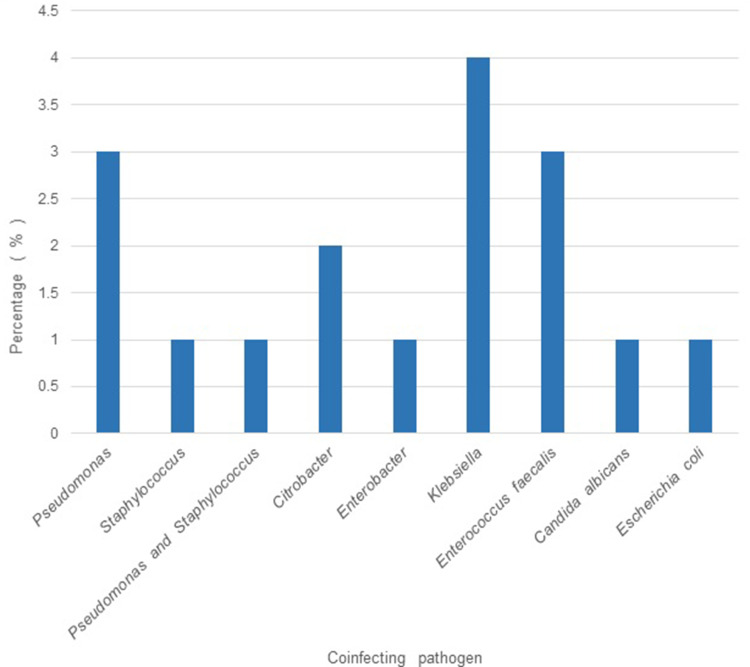
Out of the 100 isolates analyzed, 64% were of male patients and the remaining (36%), of the female. The mean age of the patients from whom the isolates were obtained was 52 years, and the maximum number of patients (26%) belonged to the age group of 60–70 years. 72% of the isolates were found to have been obtained from patients admitted in the Intensive Care Unit (ICU), whereas 21% were from various wards, and the remaining were from the Operation Theatre (OT) (5%) and Post-operative wards (2%). (Figure 1). The duration of stay ranged from 1 to 90 days, with a mean duration of 18 days. A large proportion of isolates were obtained from endotracheal (ET) suction tubes (21%), ET aspirate (14%), sputum (13%), wound swab (14%), urine (9%), and the remaining from other clinical specimens such as bronchoalveolar lavage, surgical site swab, deep tissue, bone tissue, catheter tip, pus, foot blister, tracheostomy suction tip, and abdominal drain swab (Table 1). The isolates obtained collectively from urine, deep tissue, bone tissue, and pus, which amount to 19%, are strongly inclined towards clinical infection rather than mere colonization. The risk factors observed to be present at relatively high frequencies include mechanical ventilation (62%), diabetes mellitus (30%), tracheostomy (27%), and duration of stay > 15 days (46%), age > 50 years (63%). A high proportion of isolates (>50%) showed resistance independently to imipenem and meropenem, while 43% showed resistance to ertapenem (Figure 2). Amongst other antibiotics to which >50% isolates showed resistance are gentamicin, ciprofloxacin, ampicillin, and piperacillin/tazobactam. Higher rates of susceptibility were observed with a few antibiotics such as tigecycline (55%). 61% of the patients were given appropriate antibiotics against Acinetobacter colonization/infection, whereas 38% received no specific treatment, and 1% had to undergo amputation due to severe disease. Concerning the clinical outcomes of the patients with colonization/infection, 87% were discharged, 12% expired, and 1% were shifted to a different hospital for further treatment. A positive correlation was found between the duration of hospital stay and number of antibiotics to which the isolate was resistant (p = 0.013). Isolates obtained from patients in the ICU were found to show resistance to a larger number of antibiotics compared to those in the wards and OT. (p=0.023) (Table 2) There was no significant association established between the number of antibiotics and other risk factors such as age, tracheostomy, mechanical ventilation, catheterization, diabetes mellitus, the source of isolate, etc. Eighty-three (83) % of the isolates did not show the presence of a co-infecting pathogen whereas in the remaining cases, organisms such as Pseudomonas, Enterococcus, Klebsiella, etc. were present (Figure 3). There was no significant variation in the outcome of the patients based on the number of antibiotics to which the isolate showed resistance (Table 3). There was no significant difference in the antibiotic resistance patterns among the various sources of isolates.
Figure 1.
(N = 100): Percentage of multidrug resistant isolates obtained based on the section of the hospital where the patient was when the sample was collected.
Table 1.
Frequency of Multidrug Resistant Isolates Obtained from Various Sources (N=100)
| Frequency | Percent | |
|---|---|---|
| Sputum | 13 | 13.0 |
| ET suction | 21 | 21.0 |
| ET aspirate | 14 | 14.0 |
| BAL | 8 | 8.0 |
| Wound swab | 3 | 3.0 |
| Surgical site | 1 | 1.0 |
| Central line | 3 | 3.0 |
| Urine | 9 | 9.0 |
| Deep tissue | 4 | 4.0 |
| Bone tissue | 2 | 2.0 |
| Catheter tip | 1 | 1.0 |
| Pus | 4 | 4.0 |
| Foot blister | 1 | 1.0 |
| Tracheostomy suction tip | 3 | 3.0 |
| Abdominal drain swab | 2 | 2.0 |
| Swab | 11 | 11.0 |
| Total | 100 | 100.0 |
Abbreviations: ET, endotracheal tube; BAL, bronchoalveolar lavage.
Figure 2.
(N = 100): Percentage of isolates showing resistance to particular antibiotics.
Table 2.
Number of Antibiotics to Which Isolates from Various Sections of the Hospital Showed Resistance (N=100) (p=0.023)
| Section | Total | ||||||
|---|---|---|---|---|---|---|---|
| ICU | Post-Op Ward | Ward | OT | ||||
| Number of antibiotics to which isolate is resistant | 1–5 | Count | 1 | 0 | 4 | 0 | 5 |
| % | 1.4% | 0.0% | 19.0% | 0.0% | 5.0% | ||
| 6−7 | Count | 9 | 1 | 5 | 0 | 15 | |
| % | 12.5% | 50.0% | 23.8% | 0.0% | 15.0% | ||
| 8−9 | Count | 16 | 0 | 6 | 4 | 26 | |
| % | 22.2% | 0.0% | 28.6% | 80.0% | 26.0% | ||
| 10–11 | Count | 10 | 1 | 0 | 0 | 11 | |
| % | 13.9% | 50.0% | 0.0% | 0.0% | 11.0% | ||
| 12−13 | Count | 19 | 0 | 4 | 0 | 23 | |
| % | 26.4% | 0.0% | 19.0% | 0.0% | 23.0% | ||
| 14−15 | Count | 12 | 0 | 1 | 1 | 14 | |
| % | 16.7% | 0.0% | 4.8% | 20.0% | 14.0% | ||
| ≥16 | Count | 5 | 0 | 1 | 0 | 6 | |
| % | 6.9% | 0.0% | 4.8% | 0.0% | 6.0% | ||
| Total | Count | 72 | 2 | 21 | 5 | 100 | |
| % | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | ||
Abbreviations: ICU, intensive care unit; Post-op ward, post operative ward; OT, operation theatre.
Figure 3.
(N = 100): Percentage of coinfecting pathogens.
Table 3.
Clinical Outcome v/s the Number of Antibiotics the Isolate Showed Resistance to (N=100) (p=0.317)
| Outcome | Total | |||||
|---|---|---|---|---|---|---|
| Discharged | Death | Shifted | ||||
| Number of antibiotics to which isolate is resistant | 1–5 | Count | 4 | 1 | 0 | 5 |
| % | 4.6% | 8.3% | 0.0% | 5.0% | ||
| 6−7 | Count | 15 | 0 | 0 | 15 | |
| % | 17.2% | 0.0% | 0.0% | 15.0% | ||
| 8−9 | Count | 23 | 3 | 0 | 26 | |
| % | 26.4% | 25.0% | 0.0% | 26.0% | ||
| 10–11 | Count | 7 | 3 | 1 | 11 | |
| % | 8.0% | 25.0% | 100.0% | 11.0% | ||
| 12−13 | Count | 21 | 2 | 0 | 23 | |
| % | 24.1% | 16.7% | 0.0% | 23.0% | ||
| 14−15 | Count | 12 | 2 | 0 | 14 | |
| % | 13.8% | 16.7% | 0.0% | 14.0% | ||
| ≥16 | Count | 5 | 1 | 0 | 6 | |
| % | 5.7% | 8.3% | 0.0% | 6.0% | ||
| Total | Count | 87 | 12 | 1 | 100 | |
| % | 100.0% | 100.0% | 100.0% | 100.0% | ||
Discussion
Multidrug resistance in Acinetobacter baumannii is constantly on the rise. Epidemiological studies reveal that there has been a rise in the mortality rates of infections associated with Acinetobacter baumannii, and currently ranges from 10% to 43% in ICUs and from 7.8% to 23% outside ICUs.17 Several other retrospective studies conducted in the USA show a significant positive association between mortality and colonization with MRAB.18,19 Acinetobacter baumannii is a ubiquitous microbe and can be commonly isolated from hospital environments and hospitalized patients, especially in the ICU. In a retrospective study conducted at a university hospital in France, with an observation period of 4 years, it was observed that multidrug-resistant strains were more likely to be isolated from ICU samples than susceptible ones.20 Our study too showed a higher frequency of isolates from patients admitted in the ICU (76%) (Figure 1). A recent survey conducted in Chattisgarh showed a significant association of risk factors such as Ischemic heart disease and mechanical ventilation with MDR Acinetobacter and also showed a higher degree of resistance in ICU samples.21 Various other studies across the globe have shown significant association with mechanical ventilation, ICU stay, urinary catheterization, endotracheal tube, central venous catheterization, etc.22 In our study, we observed that the number of antibiotics to which the isolates were resistant to, was significantly more abundant in isolates obtained from the ICU compared to those from wards and OT (Table 2). But there was no significant association observed with the other risk factors. We also noticed a significant positive correlation between the duration of hospital stay and the number of antibiotics to which the isolates showed resistance (p = 0.013). Previous studies have shown a significant increase in mortality in cases of infection with Multidrug-resistant Acinetobacter baumannii. However, we did not find a significant association between the antibiotic resistance levels and the clinical outcomes of the patients. Carbapenems have been first-line antibiotics for this organism, but several studies have shown that there has been a rapid rise in the development of resistance to this group of drugs. A retrospective study conducted at a hospital in Poland in 2018, with a six-year observation period (2011–2016) revealed a 3-fold rise in the incidence of Acinetobacter baumannii infections and a significant increase in resistance to carbapenems.23 An increase in the rate of isolation of Multidrug-resistant strains of Acinetobacter baumannii, with high resistance to carbapenems, has also been observed in studies conducted in the past decade in various parts of North India.24,25 In our study, a high proportion of isolates (>50%) showed resistance independently to imipenem and meropenem, while 43% showed resistance to ertapenem (Figure 2) This resistance pattern is an alarming statistic as carbapenems have been considered to be a highly active group of antibiotics against Acinetobacter organisms in the past. A high degree of resistance was also seen with other antibiotics such as amikacin, gentamicin, ciprofloxacin, and piperacillin/tazobactam. While there have been such studies conducted around the world, and a few in India, there has been done in or around Mangalore, and hence the resistance patterns of Acinetobacter baumannii in this city are mostly unknown. This retrospective study showed a significantly large proportion of carbapenem-resistant isolates, thereby questioning their validity as the first-line antibiotics against infection by this organism. The multi-locus sequence typing (MLST) of isolates during the outbreak could contribute to the existing knowledge and know the distribution of type of clone in various geographical locations. Whole Genome Sequencing (WGS) studies can differentiate bacteria that have identical resistance patterns caused by different mechanisms. This includes the full complement of resistance determinants, including resistance to compounds not routinely tested phenotypically. In the recent past, BacWGSTdb, a technology based on usage of combination of data of WGS, source, geographical location, host, disease, the closely related species clone or new clones, the location and other attributes can easily be recognized. This tool can play a crucial role in analyzing the data of drug-resistant organisms like Acinetobacter. The online database provides a rapid, low cost and suitable tool for tackling the menace of antimicrobial resistance and also contribute to global AMR surveillance. The concept of ideas of WGS with the other attributes, when put into routine use could move from bench to bedside in day to day clinical practice.26
Conclusion
This was a retrospective study conducted using the hospital records of multidrug-resistant Acinetobacter baumannii isolates of patients admitted in two tertiary hospitals in the city of Mangalore. The risk factors found to be significantly associated were ICU stay, and duration of hospital stay. Whereas other factors such as mechanical ventilation, tracheostomy, diabetes mellitus, catheterization, central line insertion were not found to have a significant association. A large proportion of the organisms were found to show resistance to carbapenems such as imipenem, meropenem, and ertapenem, thereby signifying the need for further research and use of other antibiotics such as tigecycline, to which a higher rate of susceptibility was found.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. Pharm Ther. 2015;40(4):277. [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz RS. Paul Ehrlich’s magic bullets. N Engl J Med. 2004;350(11):1079–1080. doi: 10.1056/NEJMp048021 [DOI] [PubMed] [Google Scholar]
- 3.Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: the emergence of a successful pathogen. Clin Microbiol Rev. 2008;21(3):538–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howard A, O’dounoghue M, Feeney A, Sleeator RD. Acinetobacter baumannii: an emerging opportunistic pathogen. Virulence. 2012;3(3):243–250. doi: 10.4161/viru.19700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asif M, Alvi IA, Rehman SU. Insight into Acinetobacter baumannii: pathogenesis, global resistance, mechanisms of resistance, treatment options, and alternative modalities. Infect Drug Resist. 2018;11:1249. doi: 10.2147/IDR.S166750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al Atrouni A, Joly-Guillou ML, Hamze M, Kempf M. Reservoirs of non-baumannii Acinetobacter species. Front Microbiol. 2016;7:49. doi: 10.3389/fmicb.2016.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernández L, Hancock RE. Adaptive and mutational resistance: role of porins and efflux pumps in drug resistance. Clin Microbiol Rev. 2012;25(4):661–681. doi: 10.1128/CMR.00043-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alsan M, Klompas M. Acinetobacter baumannii: an emerging and important pathogen. J Clin Outcomes Manag. 2010;17(8):363. [PMC free article] [PubMed] [Google Scholar]
- 9.Manchanda V, Sanchaita S, Singh NP. Multidrug resistant acinetobacter. J Glob Infect Dis. 2010;2(3):291. doi: 10.4103/0974-777X.68538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergogne-Berezin E, Towner KJ. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin Microbiol Rev. 1996;9(2):148. doi: 10.1128/CMR.9.2.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rello J, Diaz E. Acinetobacter baumannii: a threat for the ICU? Intensive Care Med. 2003;29(3):350–351. doi: 10.1007/s00134-003-1661-y [DOI] [PubMed] [Google Scholar]
- 12.Sileem AE, Said AM, Meleha MS. Acinetobacter baumannii in ICU patients: a prospective study highlighting their incidence, antibiotic sensitivity pattern and impact on ICU stay and mortality. Egypt J Chest Dis Tuberc. 2017;66(4):693–698. doi: 10.1016/j.ejcdt.2017.01.003 [DOI] [Google Scholar]
- 13.Dent LL, Marshall DR, Pratap S, Hulette RB. Multidrug resistant Acinetobacter baumannii: a descriptive study in a city hospital. BMC Infect Dis. 2010;10:196. doi: 10.1186/1471-2334-10-196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altun HU, Yagci S, Bulut C, et al. Antimicrobial susceptibilities of clinical Acinetobacter baumannii isolates with different genotypes. Jundishapur J Microbiol. 2014;7(12). doi: 10.5812/jjm.13347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eliopoulos GM, Maragakis LL, Perl TM. Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin Infect Dis. 2008;46(8):1254–1263. doi: 10.1086/529198 [DOI] [PubMed] [Google Scholar]
- 16.Ye JJ, Huang CT, Shie SS, et al. Multidrug resistant Acinetobacter baumannii: risk factors for appearance of imipenem resistant strains on patients formerly with susceptible strains. PLoS One. 2010;5(4):e9947. doi: 10.1371/journal.pone.0009947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blanco N, Harris AD, Rock C, et al. Risk factors and outcomes associated with multidrug-resistant Acinetobacter baumannii upon intensive care unit admission. Antimicrob Agents Chemother. 2018;62(1):e01631–17. doi: 10.1128/AAC.01631-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abbo A, Carmeli Y, Navon-Venezia S, Siegman-Igra Y, Schwaber MJ. Impact of multi-drug-resistant Acinetobacter baumannii on clinical outcomes. Eur J Clin Microbiol Infect Dis. 2007;26(11):793–800. doi: 10.1007/s10096-007-0371-8 [DOI] [PubMed] [Google Scholar]
- 19.Fillaux J, Dubouix A, Conil J-M, Laguerre J, Marty N. Retrospective analysis of multidrug-resistant acinetobacter baumannii strains isolated during a 4-year period in a University Hospital. Infect Control Hosp Epidemiol. 2006;27(7):647–653. doi: 10.1086/507082 [DOI] [PubMed] [Google Scholar]
- 20.Duszynska W, Litwin A, Rojek S, Szczesny A, Ciasullo A, Gozdzik W. Analysis of Acinetobacter baumannii hospital infections in patients treated at the intensive care unit of the University Hospital, Wroclaw, Poland: a 6-year, single-center, retrospective study. Infect Drug Resist. 2018;11:629. doi: 10.2147/IDR.S162232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung JY, Park MS, Kim SE, et al. Risk factors for multidrug resistant Acinetobacter baumannii bacteremia in patients with colonization in the intensive care unit. BMC Infect Dis. 2010;10(1):228. doi: 10.1186/1471-2334-10-228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munier AL, Biard L, Rousseau C, et al. Incidence, risk factors, and outcome of multidrug-resistant Acinetobacter baumannii acquisition during an outbreak in a burns unit. J Hosp Inf. 2017;97(3):226–233. doi: 10.1016/j.jhin.2017.07.020 [DOI] [PubMed] [Google Scholar]
- 23.Odsbu I, Khedkar S, Khedkar U, Nerkar SS, Tamhankar AJ, Stålsby Lundborg C. High proportions of multidrug-resistant Acinetobacter spp. isolates in a district in Western India: a four-year antibiotic susceptibility study of clinical isolates. Int J Environ Res Public Health. 2018;15(1):153. doi: 10.3390/ijerph15010153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khajuria A, Praharaj AK, Kumar M, Grover N. Molecular characterization of carbapenem resistant isolates of Acinetobacter baumannii in an intensive care unit of a tertiary care centre at central India. J Clin Diagn Res. 2014;8(5):DC38. doi: 10.7860/JCDR/2014/6788.3956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sherwani N, Barapatre R, Neral A, Singh R, Gade N. A study of multidrug-resistant Acinetobacter baumannii – a susceptibility pattern and possible risk factors in a tertiary care hospital of Chattisgarh. J Evol Med Dent Sci. 2018;7(36):3935–3940. doi: 10.14260/jemds/2018/880 [DOI] [Google Scholar]
- 26.Ruan Z, Feng Y. BacWGSTdb, a database for genotyping and source tracking bacterial pathogens. Nucleic Acids Res. 2016;44(D1):D682–D687. doi: 10.1093/nar/gkv1004 [DOI] [PMC free article] [PubMed] [Google Scholar]