Abstract
Background
Gynecologic cancers are associated with high rates of venous thromboembolism (VTE), which is exacerbated by pelvic surgery and chemotherapy.
Objectives
The aim of this study was to develop and validate a risk score for VTE in patients with gynecologic cancer and to test the predictive ability of the score following addition of procoagulant biomarker data.
Patients and methods
Clinical and laboratory variables were used to develop a risk score for the prediction of VTE in patients with gynecological cancer (n = 616), which was validated in a separate cohort of patients (n = 406). Endogenous thrombin potential and D‐dimer levels were determined in a subset (n = 290) of patients and used to produce an extended score in the validation cohort.
Results
Multivariable regression analysis identified BMI >30, hemoglobin <11.5 g/dL and chemotherapy as independent predictors of VTE, which formed the Thrombogyn score. Following competing risk regression analysis, subdistribution hazard ratios (SHRs), adjusted for cancer stage, were 8.16 (95% confidence interval [CI], 1.69‐43.77) in the high‐risk group (score = 2‐3) and 4.12 (95% CI, 0.85‐20.15) in the intermediate‐risk group (score = 1) compared with the low‐risk group (score = 0). SHRs for the validation cohort were 6.26 (95% CI, 1.24‐31.39) and 3.00 (95% CI, 0.67‐13.32), respectively. Cumulative incidence of VTE in the validation cohort high‐risk group was 10.34% (95% CI, 6.51‐16.41) per women‐years compared with 1.06% (95% CI, 0.26‐4.26) in the low‐risk group. Using the extended Thrombogyn score, adjusted SHRs were 16.83 (95% CI, 4.20‐67.37) in the high‐risk group with a cumulative incidence of 21.15% (95% CI, 10.32‐45.24). External validation of the score is required.
Conclusions
The Thrombogyn score identifies patients with gynecologic cancer at high and low risk of VTE. Addition of biomarker data improves the predictive power of the score.
Keywords: biomarkers, cancer, risk, thrombin, venous thromboembolism, women
Essentials.
Patients with gynecologic cancer are at high risk of venous thromboembolism (VTE).
Thrombogyn risk score for VTE was derived and validated in 1022 patients with gynecologic cancer.
The addition of procoagulant biomarkers increases the predictive power of the Thrombogyn score.
1. INTRODUCTION
Gynecologic cancers are associated with a high risk of venous thromboembolism (VTE). 1 , 2 , 3 Patients are particularly at risk during the postoperative period, where VTE occurs in 6%‐7% of patients with gynecologic cancer after surgery despite prophylaxis. 1 The risk of pulmonary embolism (PE) is increased 14‐fold in patients undergoing gynecologic cancer surgery compared with those who had similar surgery for benign disease. 4 In line with these findings, guidelines advise extended low‐molecular‐weight heparin (LMWH) prophylaxis following surgery for gynecologic cancers beyond the patient’s hospital stay. 5 , 6 Although extended prophylaxis is effective, 7 the optimal duration of prophylaxis is unclear. 8 , 9 Several groups have suggested that extended prophylaxis is not necessary in all patients with gynecologic cancer, particularly those undergoing minimally invasive surgery. 10 , 11 , 12 In addition, we and others have shown that use of extended prophylaxis is low in many centers. 2 , 13 , 14 , 15
Thrombosis is also a common complication of chemotherapy, particularly among patients with ovarian cancer. 16 , 17 Two recent randomized trials showed that chemotherapy‐associated VTE can be reduced using prophylaxis with direct oral anticoagulants in intermediate to high‐risk patients. 18 , 19 For optimal prevention of VTE in patients with cancer, effective risk assessment strategies are required. 20
Several models have been developed to identify patients with cancer at high risk of VTE. The most widely used and externally validated of these, the Khorana risk score, was developed to identify patients who are at increased risk of VTE during chemotherapy. 21 Similar risk models using a combination of clinical risk factors and biomarkers have been published, although few have been validated. 22 , 23 , 24 , 25 The major determinant of the Khorana score, and the most recent score from the Vienna Cancer Associated Thrombosis cohort, 26 is cancer site, which places all patients with gynecologic cancer in an intermediate‐risk category prior to the addition of other risk determinants. Hence, this approach lacks stratification power among cohorts of a single cancer type, as has been shown in studies of patients with lung cancer. 27 , 28 Patients with gynecologic cancer are poorly represented in these studies, which limits the applicability of the scores to these patients.
In this study, we have developed and validated the Thrombogyn score, a risk score for VTE in patients with gynecologic cancer undergoing surgery and chemotherapy. The predictive ability of extending the Thrombogyn score with procoagulant‐based biomarkers was also determined.
2. STUDY DESIGN
Patients who underwent surgery for gynecologic cancer from January 2006 to June 2016 in St. James Hospital gynecology‐oncology unit (a large tertiary referral center) in Dublin were included in the study. Patients who were on LMWH treatment for VTE (including cancer‐related VTE) at the time of surgery were excluded. Patients were not screened for thrombophilia, but patients with known thrombophilia requiring additional prophylaxis were excluded. Patients on long‐term anticoagulant therapy for other conditions were also excluded. All patients were treatment naïve except for patients who had neoadjuvant therapy or patients who underwent surgery for recurrence. All patients received antithrombotic prophylaxis following surgery. This included early mobilization, hydration, compression stockings, and LMWH (4500 IU tinzaparin once daily (body mass index [BMI], < 40 kg/m2) and 75 IU/kg tinzaparin once daily for BMI > 40 kg/m2. Patients were followed for a minimum of 24 months or until VTE occurrence or death. The study had the approval of the local ethics committee.
The primary end point was objectively confirmed VTE, including deep vein thrombosis (DVT), PE, or both (DVT and PE) following cancer surgery. Only events that were confirmed by documented objective testing such as compression ultrasonography, venography, or computed tomography and pulmonary angiogram in the case of PE, were included. Although the majority of events were symptomatic, asymptomatic thrombotic events (eg, PE detected in a routine computed tomography), were also included when these events required treatment. Sample size was determined based on the rule of thumb that 10 events per predictor are required for a valid analysis. 29 Assuming an average VTE rate for gynecologic patients of 7%, 1 a sample size of 700 patients (50 VTE events) would be enough for a risk score with up to 5 predictors.
The derivation cohort consisted of all patients who underwent surgery between January 2006 and June 2012 and who fulfilled the inclusion/exclusion criteria. Data were extracted retrospectively from the gynecologic cancer database, with follow‐up data obtained from hospital and general practitioner records. Patients in the derivation cohort received LMWH prophylaxis for the duration of their hospital stay.
The validation cohort consisted of patients with gynecologic cancer who underwent surgery between May 2012 and June 2016 and who donated samples prospectively to the Trinity College Dublin (TCD) gynecologic cancer bioresource, a prospectively collected database and biobank of tissue, serum, and plasma from ovarian, endometrial, and other gynecologic cancers. All patients were prescribed LMWH prophylaxis for 4 weeks after surgery in accordance with the guidelines. 5 , 6 All patients gave full and informed written consent.
For both cohorts, patient age, BMI, final histologic diagnosis, tumor origin, International Federation of Gynecology and Obstetrics stage and grade of cancer 30 , 31 surgical approach (open/laparoscopic), surgical complexity, duration of hospital stay, chemotherapy and radiotherapy treatment, and survival data were recorded. Preoperative hemoglobin (Hb), white cell, neutrophil, lymphocyte, and platelet counts were determined 24‐48 hours prior to surgery by the hospital laboratory using standard procedures. Surgical procedures were classified according to complexity of surgery using a modification of a previously described classification system 32 (Table S1). Duration of follow‐up was calculated from date of surgery to date of last follow‐up, VTE, or death.
2.1. Derivation and validation of the Thrombogyn score
The Thrombogyn score was developed based on data from the derivation cohort only. Variables included were age; BMI; tumor origin, stage and grade of cancer, histology, chemotherapy treatment, radiotherapy treatment, and surgical complexity and approach; and Hb, white cell, lymphocyte, neutrophil, and platelet count. Categorical variables were evaluated in univariate analysis using a chi‐squared test, and continuous variables were analyzed using a Student t test. Variables associated with an increased risk of VTE in univariate analysis (P < .2) as well as continuous variables that showed a significant difference between VTE and non‐VTE groups were selected for inclusion in the multivariable analysis. Stepwise forward multivariable binary logistic regression was used with VTE occurrence during follow‐up set as the dependent variable. A risk score was developed based on the regression coefficients (β) of the independent predictors rounded to the nearest integer. Each patient was scored using the derived score; smaller score groups were combined to produce 3 categories: low, medium, and high risk. The score was validated using a separate cohort from the TCD gynecologic cancer bioresource (described above).
2.2. Laboratory analysis
Platelet‐poor plasma prepared as previously described 33 was obtained from the TCD gynecologic cancer bioresource. Blood samples were taken 24‐48 hours prior to surgery. D‐dimer was measured using a 2‐step procedure in the hospital laboratory (Vidas D‐dimer Exclusion II). Thrombin generation (Thrombinoscope, Synapse BV), was measured as previously described using 5 PM Tissue Factor. 33 Area under the thrombin generation curve (ETP) was reported for each sample.
2.3. Extension of the Thrombogyn score with addition of biomarker data
The score was extended in the validation cohort with the addition of procoagulant biomarkers (ETP) and D‐dimer. ETP and D‐dimer levels were determined in all available samples. A predefined cutoff of the 75th percentile for each biomarker was calculated, which reflected the upper quartile of the total biobank study population. Values above the 75th percentile were assigned a score of 1 point for each biomarker and added to the Thrombogyn score, creating a 5‐point extended Thrombogyn score. Smaller score groups were combined to produce a low‐, intermediate‐, and high‐risk group.
2.4. Statistical analysis
Model discrimination performance was evaluated using the standard measures of sensitivity, specificity, and predictive value. For overall assessment, discrimination was evaluated using the area under the receiver operating characteristic curve (AUC) with larger values indicating better discrimination. Calibration was assessed by plotting observed versus predicted probability of VTE in each cohort. The cumulative VTE incidence rate was estimated using a competing risk time‐to‐event analysis in which death was treated as a competing event for VTE. Subdistribution hazard ratios (SHRs) adjusted for competing risk of death were calculated with the Thrombogyn score as a continuous variable. SHRs (adjusted for cancer stage) were also calculated for each risk category, with the low‐risk category as a reference. Kaplan‐Meier curves were used to determine thrombosis‐free survival for each risk category. In all cases, P < .05 was considered significant. Data were analyzed using SPSS version 23(IBM Corporation, Armonk, NY, USA) and Stata version 13.1 (Stata Corporation, College Station, TX, USA).
3. RESULTS
3.1. Derivation of the Thrombogyn score
A total of 802 patients were reviewed, and 620 patients met the inclusion criteria. Reasons for exclusion included preoperative VTE, long‐term anticoagulation, no surgery, or surgery in another hospital (Figure 1). Four patients were lost to follow‐up. Ovarian cancer (51.8%), endometrial cancer (28.4%), and cervical cancer (13.8%) were the main cancer sites. A total of 554 (89.9%) patients were treatment naïve at inclusion; 229 (37.2%) patients had adjuvant chemotherapy during the follow‐up period (full demographic details are given in Table 1).
Figure 1.
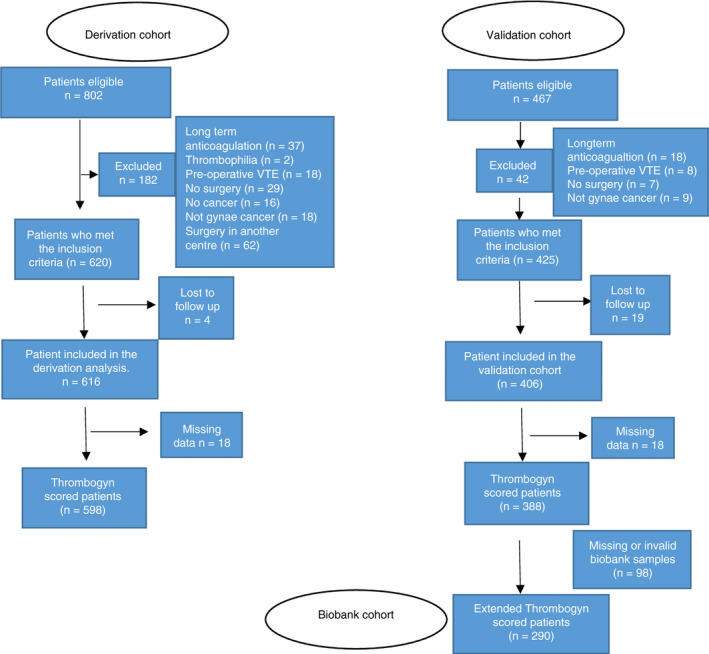
Flow diagram showing inclusion of patients into the study
Table 1.
Demographics of the population
| Derivation cohort (n = 616) | Validation cohort (n = 406) | P value | ||
|---|---|---|---|---|
| Age, y, median (IQR) | 57 (46‐66.0) | 59 (50‐66) | < .02 | |
|
Tumor site, n (%) |
Ovary | 319 (51.8) | 135 (33.3) | < .001 |
| Endometrium | 175 (28.4) | 187 (46.1) | ||
| Cervix | 85 (13.8) | 66 (16.3) | ||
| Other | 37 (6.0) | 18 (4.4) | ||
|
Histology, n (%) |
Clear cell | 22 (3.6) | 13 (3.2) | < .001 |
| Serous | 181 (29.4) | 109 (26.9) | ||
| Mucinous | 17 (2.8) | 12 (3.0) | ||
| Endometrioid (ovarian) | 19 (3.1) | 16 (3.1) | ||
| Endometrial | ||||
| Adenocarcinoma | 131 (21.3) | 146 (36.0) | ||
| Squamous | 90 (14.6) | 47 (11.6) | ||
| Sarcomas | 16 (2.6) | 20 (4.9) | ||
| Mixed | 25 (4.1) | 19 (4.7) | ||
| Borderline | 67 (10.9) | 0 | ||
| Other | 48 (7.8) | 24 (5.9) | ||
|
Stage, n (%) |
I | 287 (46.6) | 224 (55.2) | < .001 |
| II | 43 (7.0) | 32 (7.9) | ||
| III | 175 (28.4) | 90 (22.2) | ||
| IV | 41 (6.6) | 48 (11.8) | ||
| Recurrent | 3 (0.5) | 12 (3.0) | ||
| N/A | 67 (10.8) | 0 | ||
|
Grade, n (%) |
I | 149 (24.1) | 125 (30.8) | < .001 |
| II | 137 (22.2) | 108 (26.6) | ||
| III | 254 (41.2) | 161 (39.7) | ||
| Recurrent | 3 (0.5) | 12 (3.0) | ||
| N/A | 73 (11.8) | 0 | ||
|
BMI, n (%) |
>30 kg/m2 | 187 (30.3) | 174 (42.8) | < .001 |
| <30 kg/m2 | 411 (66.7) | 214 (52.7) | ||
| N/A | 18 (2.9) | 18 (4.4) | ||
|
Chemotherapy, n (%) |
Neoadjuvant | 62 (10.1) | 44 (10.8) | .543 |
| Adjuvant | 229 (37.2) | 139 (34.2) | ||
| No chemotherapy | 325 (52.8) | 223 (54.9) | ||
|
Radiotherapy, n (%) |
Yes | 135 (21.9) | 131 (32.3) | < .001 |
| No | 367 (59.6) | 264 (65.0) | ||
| N/A | 38 (6.2) | 57 (14.0) | ||
|
Surgical complexity, n (%) |
Low | 211 (34.3) | 131 (32.3) | < .0001 |
| Intermediate | 367 (59.6) | 218 (53.7) | ||
| High | 38 (6.2) | 57 (14.0) | ||
| Duration of hospital stay, days (median (range)) | 10 (7‐14) | 7 (5‐9) | < .0001 | |
| VTE, n (%) | 53 (8.6) | 34 (8.4) | .753 | |
|
Surgical approach, n (%) |
Open | 540 (87.6) | 226 (55.7) | < .0001 |
| Laparoscopic | 76 (12.3) | 180 (44.3) | ||
| White cell count (×103/μL), median (IQR) | 7.5 (6.1‐9.2) | 7.3 (5.8‐8.9) | .836 | |
| Neutrophils (×103/μL), median (IQR) | 4.7 (3.5‐6.4) | 4.5 (3.5‐6.1) | .384 | |
| Lymphocytes (×103/μL), median (IQR) | 1.8 (1.3‐2.3) | 1.8 (1.4‐2.3) | .358 | |
| Hemoglobin (g/dL), median (IQR) | 12.5 (11.1‐13.4) | 13.0 (11.7‐13.9) | < .001 | |
| Platelets (×103/μL), median (IQR) | 281 (235‐345) | 287 (239‐347) | .832 | |
VTE occurred in 53 patients in the derivation cohort (3 patients were asymptomatic). VTE events were PE (n = 21), DVT (n = 23), DVT and PE (n = 7), peripherally inserted central catheter (PICC) line thrombosis (n = 1), and subclavian and axillary vein thrombosis (n = 1). Eleven (20.7%) VTEs occurred during chemotherapy treatment. The median time to VTE event from surgery was 104 (1‐720) days. Twenty‐three (43.4%) events occurred within 1 month of surgery, including 12 (22.6%) VTE events that occurred during hospital stay while patients were on LMWH prophylaxis. Two patients who had a VTE within 30 days of surgery had an intraoperative bleed requiring transfusion.
Univariate analysis of potential risk factors is summarized in Table S2. The following variables were included in the multivariable analysis: tumor histology, stage, and grade; BMI; surgical complexity; duration of hospital stay; chemotherapy treatment; and Hb (<11.5 g/dL). Multivariable regression analysis showed that BMI > 30, chemotherapy treatment, and Hb < 11.5 g/dL were independently associated with VTE. The analysis was repeated for VTE events occurring within 12 months of surgery, and the same independent predictors were identified. Regression coefficients and odds ratios for each predictor are shown in Table 2 and Table S3.
Table 2.
Multivariable analysis results for the derivation cohort (n = 616) showing independent predictors that comprise the Thrombogyn score
| Beta | SE | df | P value | OR | 95% CI | No. of points assigned | |
|---|---|---|---|---|---|---|---|
| Hemoglobin < 11.5 | 0.941 | 0.308 | 1 | .002 | 2.56 | 1.41‐4.67 | 1 |
| BMI > 30 | 0.652 | 0.318 | 1 | .04 | 1.92 | 1.03‐3.57 | 1 |
| Chemotherapy | 1.316 | 0.344 | 1 | .0001 | 3.73 | 1.90‐7.32 | 1 |
Points are assigned based on the rounding of the regression coefficient to the nearest integer.
BMI, body mass index; CI, confidence interval; df, degrees of freedom; OR, odds ratios; SE, standard error.
A total of 598 patients had complete data for scoring and were divided into 3 categories based on their scores: low risk (score = 0; n = 152), intermediate risk (score = 1; n = 264), high risk (score = 2‐3; n = 182). In the low‐risk group, 1.3% of patients suffered a VTE compared with 17.6% of patients in the high‐risk group (P < .0001) (Figure 2). Competing risk regression analysis showed that the cumulative incidence rate was 11.10% (95% confidence interval [CI], 7.85‐15.69 in the high‐risk group, compared with 0.65% (95% CI, 0.16‐2.62) in the low‐risk group (Table 3). The SHR per 1‐point increase in Thrombogyn score was 1.71 (95% CI, 0.92‐3.14), and following adjustment for cancer stage, SHR was 8.16 (95% CI, 1.69‐43.77) in the high‐risk group and 4.12 (95% CI, 0.85‐20.15) in the intermediate‐risk group (Table 3) with the low‐risk group as reference. Thrombosis‐free survival was reduced in the intermediate‐ and high‐risk groups (Figure 3A) (P < .0001).
Figure 2.

VTE rate (%) according to Thrombogyn risk score group in the derivation cohort (n = 598) and the validation cohort (n = 388). Risk groups in the biobank cohort (n = 290) are based on the extended Thrombogyn score. VTE, venous thromboembolism
Table 3.
Cumulative incidence and subdistribution hazard ratios (SHRs) for VTE following competing risk regression analysis
| Derivation cohort | Validation cohort | Extended thrombogyn cohort | |
|---|---|---|---|
| Number of scored patients | 598 | 388 | 290 |
| SHR (per point increase) | 1.71 (0.92‐3.14) | 2.47 (1.70‐3.57) | 2.17 (1.63‐2.88) |
| No. of deaths | 78 | 35 | 27 |
| Risk group | Low | Intermediate | High | Low | Intermediate | High | Low | Intermediate | High |
|---|---|---|---|---|---|---|---|---|---|
| n= | 152 | 265 | 182 | 91 | 195 | 102 | 151 | 120 | 19 |
| SHR (risk category)(95% CI) | REF | 5.79 (1.35‐24.86) | 14.85 (3.56‐61.70) | REF | 3.49 (0.81‐15.15) | 9.16 (2.16‐38.81) | REF | 4.87 (1.38‐17.17) | 20.81 (5.56‐77.85) |
| SHR(adjusted for stage)(95%CI) | REF | 4.12 (0.85‐20.15) | 8.16 (1.69‐43.77) | REF | 3.00 (0.67‐13.32) | 6.26 (1.24‐31.39) | REF | 4.15 (1.13‐15.22) | 16.83 (4.20‐67.37) |
| Cumulative incidence (per women years) (95% CI) | 0.65 (0.16‐2.62) | 3.99 (2.54‐6.26) | 11.10 (7.85‐15.69) | 1.06 (0.26‐4.27) | 3.72 (2.30‐6.28) | 10.34 (6.51‐16.41) | 1.08 (0.35‐3.37) | 5.54 (3.14‐9.76) | 21.1 (10.3‐45.2) |
P values represent the comparison for VTE in the High and Intermediate risk category compared with the Low risk category. Cumulative incidence is adjusted for competing risk of death.
CI, confidence interval; VTE, venous thromboembolism.
Figure 3.
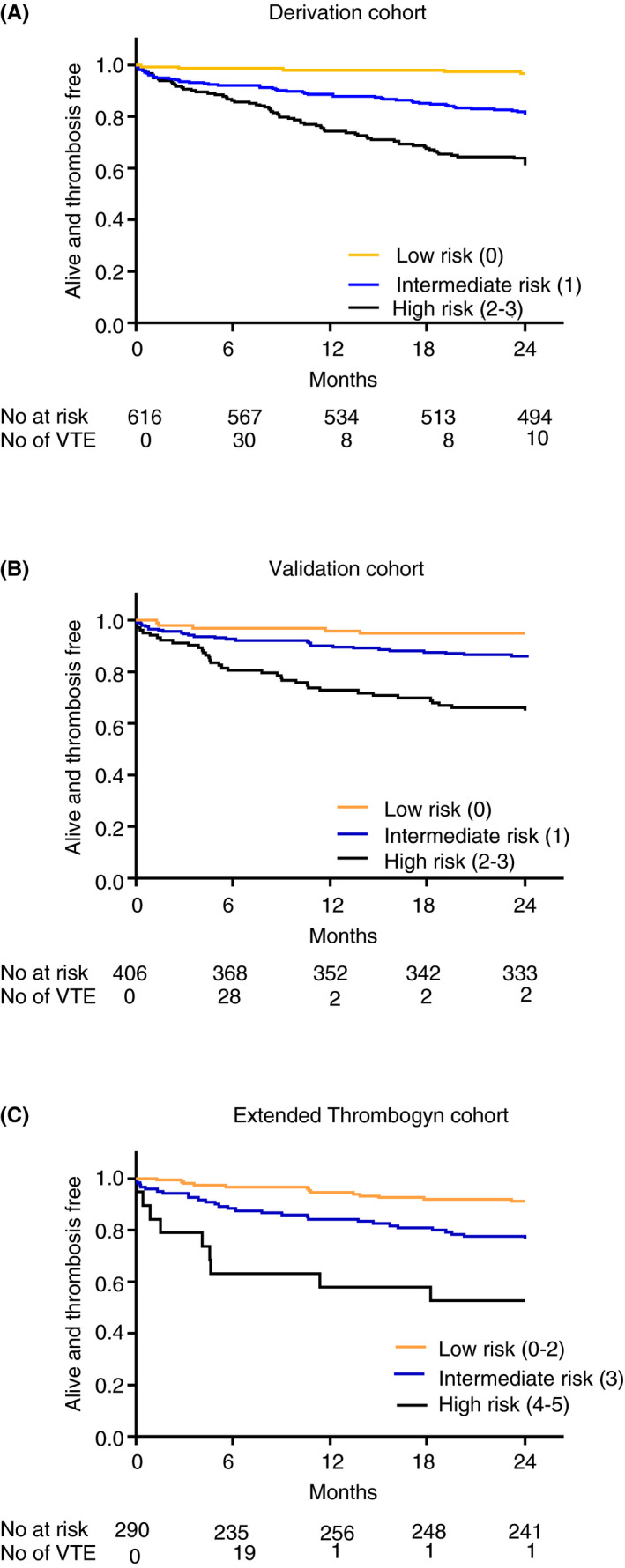
Thrombosis‐free survival according to Thrombogyn risk group in (A) derivation cohort and (B) validation cohort. (C) Thrombosis‐free survival according to extended Thrombogyn risk group in the extended Thrombogyn cohort
3.2. Validation of the score
The validation cohort contained 425 patients who met the inclusion criteria, of which 406 completed follow‐up and were included in the study; 34 of these patients suffered a VTE. Endometrial cancer was the predominant cancer site (46.1%), ovarian cancers comprised 33% of the cohort while 16% were cervical cancers. At entry to the study, 362(89.1%) patients were treatment naïve; 223 (54.9%) patients had chemotherapy during the follow‐up period. Full demographic details are given in Table 1.
Thirty‐four VTE events (5 asymptomatic) occurred during the follow‐up period (PE = 17; DVT = 15; PICC line thrombosis = 1; internal jugular vein thrombosis = 1). Eight (23.4%) VTEs occurred during chemotherapy treatment. The majority of events (82%) occurred within 12 months of follow‐up (median time to event, 68 days [range, 2‐582]). Twelve (35.2%) events occurred within 1 month of surgery (9 events during hospital stay) while on LMWH prophylaxis. Of those who suffered a VTE within 30 days of surgery, 2 patients suffered an intraoperative bleed, and 1 patient was transfused due to a low preoperative hemoglobin level.
A total of 388 patients had data available for Thrombogyn score. VTE in the high‐risk group (n = 102) was 17.6% compared with 2.2% in the low‐risk category (n = 91; P < .001) (Figure 2). VTE risk was significantly associated with Thrombogyn score with an SHR per 1‐point increase in score of 2.47 (95% CI, 1.70‐3.57) (Table 3). The adjusted cumulative incidence rates were significantly higher in both the intermediate‐ (3.72%; 95% CI, 2.20‐6.28) and high‐risk group (10.34%; 95%CI, 6.51‐16.41). Thrombosis‐free survival was reduced in the intermediate‐ and high‐risk groups, respectively, compared with the low‐risk group (P < .0001) (Figure 3B).
3.3. Addition of biomarker data to generate the extended Thrombogyn score
A total of 290 patients from the validation cohort had data available for both biomarkers (ETP and D‐dimer). Ovarian tumor site and chemotherapy were significantly less common in patients (n = 98) with missing biomarker data in the validation cohort (P < .008 and P < .014, respectively). The 75th percentile cutoff for ETP and D‐dimer in the whole population was 2475 nm thrombin/min and 1274 ng/mL, respectively (Figure S1). For values above this cutoff, 1 point was added (per biomarker) to create the 5‐point extended Thrombogyn score. D‐dimer levels above the cutoff were associated with a 3.29‐fold (95% CI, 1.27‐8.26) increased risk of VTE, whereas ETP was associated with a 2.19‐fold (95% CI, 0.89‐5.37) increased risk.
Forty‐two percent of patients in the high‐risk group (score = 4‐5; n = 19) suffered a VTE compared with 2.6% in the low‐risk group (score = 0‐2; n = 151) (P < .0001) (Figure 2). Cumulative incidence rate for the extended score was 21.15% (95% CI, 10.32‐45.24) women‐years in the high‐risk group compared with 1.08 (95% CI, 0.35‐3.37) in the low‐risk group. SHR per 1‐point increase in the extended Thrombogyn score, showed a significant association with VTE risk (SHR = 2.17 [1.63‐2.88]) (Table 3). Thrombosis‐free survival was significantly shorter in the high‐risk group compared with the low‐risk group (P < .001) (Figure 3C).
3.4. Accuracy and sensitivity of the score
AUC for the Thrombogyn score in the derivation and validation cohorts were similar, at 0.714 (95% CI, 0.645‐0.780) and 0.699 (95% CI, 0.605‐0.780), respectively (Table 4; Figure S2A,B). The sensitivity and negative predictive value (NPV) of the Thrombogyn score in the intermediate‐/high‐risk group combined (score ≥ 1) was >90% in both derivation and calibration cohorts. In the biomarker cohort, the extended Thrombogyn score had a higher AUC (0.784; 95% CI, 0.681‐0.886), higher specificity, and positive predictive value for the high‐risk group compared with the Thrombogyn score in the same patient cohort; however, sensitivity was lower (Table 5; Figure S2C). Calibration plots of observed versus predicted probability of VTE showed good agreement between observed and predicted probabilities in each cohort (Figure S2D‐F).
Table 4.
Sensitivity, specificity, and positive and negative predictive values of the Thrombogyn score in the derivation and validation cohorts
|
Sensitivity (%) (95% CI) |
Specificity (%) (95% CI) |
Positive predictive value (%) (95% CI) |
Negative predictive value (%) (95% CI) |
Area under curve (95% CI) |
|
|---|---|---|---|---|---|
| Derivation cohort | |||||
|
High risk (Thrombogyn score ≥ 2) |
60.4 (46‐73.5) |
72.4 (68.2‐76.4) |
17.6 (12.5‐24.0) |
94.9 (92.2‐96.7) |
0.714 (0.645‐0.780) |
| Intermediate/high risk (Thrombogyn score ≥ 1) |
96.2 (87.0‐99.5) |
27.9 (24.2‐31.9) |
11.4 (10.7‐12.2) |
98.7 (95.1‐99.7) |
|
| Validation cohort | |||||
|
High risk (Thrombogyn score ≥ 2) |
52.9 (35.4‐69.8) |
76.3 (72.1‐81.1) |
17.6 (11.0‐26.7) |
94.1 (90.8‐96.6) |
0.699 (0.605‐0.792) |
| Intermediate/high risk (Thrombogyn score ≥ 1) |
94.1 (80.3‐99.3) |
25.8 (21.4‐30.6) |
9.9 (9.0‐10.9) |
98.1 (92.9‐99.5) |
|
Table 5.
Sensitivity, specificity, and positive and negative predictive values of the Thrombogyn and extended Thrombogyn score in the biomarker cohort (n = 290)
|
Sensitivity (%) (95% CI) |
Specificity (%) (95% CI) |
Positive predictive value (%) (95% CI) |
Negative predictive value (%) (95% CI) |
Area under curve (95% CI) |
|
|---|---|---|---|---|---|
| Thrombogyn | |||||
|
High risk (score ≥ 2)) |
63.6 (40.6‐82.8) |
76.0 (70.4‐81.0) |
17.9 (13.0‐24.2) |
96.2 (96.5‐97.9) |
0.759 (0.658‐0.865) |
|
Intermediate/high risk (score ≥ 1) |
100 (84.6‐100.0) |
23.5 (18.5‐29.0) |
9.7 (9.1‐10.3) |
100 … |
|
| Extended Thrombogyn | |||||
|
High risk (score ≥ 4)) |
36.3 (18.0‐59.2) |
95.7 (92.5‐97.8) |
42.1 (21.1‐66.0) |
94.8 (91.2‐97.0) |
0.784 (0.681‐0.886) |
|
Intermediate/high risk (score ≥ 2) |
81.8 (59.7‐94.8) |
54.6 (48.3‐60.5) |
12.8 (10.4‐15.7) |
97.3 (93.7‐98.9) |
|
4. DISCUSSION
VTE is a significant problem for patients with gynecologic cancer occurring on average in 10% of patients over the course of their cancer journey. 1 The postoperative period is particularly high risk, 1 , 6 , 7 and for many patients, this is followed by adjuvant chemotherapy, which further increases VTE risk. 16 , 17 Although LMWH prophylaxis reduces VTE after surgery, a considerable number of patients still suffer a thrombosis despite compliance with the recommended thromboprophylaxis. 9 , 11 In this study, we have developed and validated a risk score, which can predict VTE occurrence in patients with gynecologic cancer in the 24 months following cancer staging surgery. The performance of this score was enhanced by extending the score to include 2 biomarkers (ETP and D‐dimer), known to be associated with VTE.
The postoperative period is a high‐risk period in patients with gynecologic cancer. Although further validation and randomized trials are required, the Thrombogyn score may enable clinicians to personalize the dose and duration of LMWH prophylaxis; maximizing the effectiveness of prophylaxis in high‐risk groups and identifying low‐risk patients who may be able to avoid extension of LMWH prophylaxis. The strength of this approach was recently demonstrated in 2 randomized trials where the Khorana score was used to identify high‐risk cancer patients who underwent thromboprophylaxis with direct oral anticoagulants during chemotherapy, with a significant reduction in chemotherapy‐associated VTE. 18 , 19
Several models exist for prediction of VTE in cancer; however, none are appropriate for patients with gynecologic cancer following surgery. Guidelines recommend the Caprini score to assess thrombotic risk in surgical patients; however, when this is used in gynecologic cancer surgery, >90% of patients are assessed as high risk even though only a small proportion of these developed VTE. 34 , 35 Existing scores for patients with cancer were derived based on patient cohorts that lack data on patients with gynecologic cancer. 21 , 23 , 36 The Khorana score places all patients with gynecologic cancer in the intermediate‐risk category and hence lacks the stratification required to identify lower‐risk patients. The COMPASS‐CAT risk assessment model included patients with ovarian cancer, but the majority of patients were on active treatment when assessed. 24 This limits its applicability to our patient group where approximately 90% of patients in both cohorts were treatment naïve.
In our derivation cohort, 3 risk factors emerged as independent predictors following multivariable analysis (BMI > 30, Hb < 11.5, and chemotherapy treatment) and were used to construct the Thrombogyn score. These risk factors are known to be associated with VTE in both the cancer and the noncancer population. 17 , 37
We validated our score in a separate prospective cohort from our gynecologic cancer biobank. Although the patients were from the same center, the validation cohort was significantly different from the derivation cohort (Table 1). Despite differences between the 2 cohorts and extended LMWH prophylaxis in the validation cohort, SHRs in each risk category were similar in both cohorts, which validates the Thrombogyn score.
Although our study was not designed to investigate the effects of extended prophylaxis, we found a similar incidence of VTE in the validation cohort patients (who underwent extended thromboprophylaxis) and the derivation cohort patients. Differences in risk factors between the 2 cohorts may have masked the effects of extended prophylaxis in the validation cohort. Further studies are under way in our center to investigate this in our gynecologic cancer population.
D‐dimer has been identified as the strongest prognostic biomarker for VTE in patients with cancer compared with previously tested biomarkers. 26 D‐dimer is frequently raised in ovarian cancer and has been proposed as a diagnostic marker useful in triaging patients; hence, the specificity of D‐dimer as a biomarker for VTE in this population is low. 38 Our group and others have shown that increased thrombin production as measured by the thrombin generation assay is associated with malignancy and is a predictive marker for VTE. 25 , 38 , 39 , 40 The thrombin generation assay has not been universally accepted as a biomarker in clinical settings 41 , 42 however, a recent thrombin generation standardization study showed that the standard thrombin generation assay provides good reproducibility in hypercoagulable plasma. 43
When D‐dimer and ETP are combined with the Thrombogyn score, the predictive ability of the Thrombogyn score was enhanced. The score also had improved discriminatory ability with an AUC of 0.78. This compares favorably with a similar score combining the Khorana score with D‐dimer and P‐selectin levels, which had a lower sensitivity to our score but had similar specificity and negative and positive predictive values in a mixed cancer population. 22 Similarly, addition of 2 procoagulant biomarkers to the COMPASS‐CAT score greatly improved the predictive accuracy of the score in a small cohort of mixed cancer patients. 24 , 25
Our study shows a low rate of VTE in our low‐risk group with a complete absence of VTE during the first 30 days after surgery in both validation and derivation cohorts regardless of extended prophylaxis. Our data suggest that extended prophylaxis may not be required in all patients and could be limited to a subset of patients with additional risk factors. 10 , 11 The Thrombogyn score may provide an effective tool to identify these lower‐risk patients where extended thromboprophylaxis may be avoided. This may be particularly useful for patients undergoing minimal access surgery. In addition to external validation of the Thrombogyn score, randomized trials are required to determine the effectiveness of this approach.
VTE occurred when patients were on LMWH prophylaxis (22% of VTE in the derivation cohort and 35.2% of VTE in the validation cohort). These patients were all classified by our scores as intermediate or high risk. Using the extended Thrombogyn score, the unadjusted cumulative incidence of VTE in the first 50 days after surgery in the high‐risk group was approximately 20% compared with 1% in the low‐risk group (data not shown). Forty‐two percent of patients who scored as high risk developed VTE despite extended prophylaxis. For these high‐risk patients, the recommended prophylaxis does not appear to be adequate and an alternative prophylactic strategy may be required. Although our score requires extensive external validation, the extended Thrombogyn score may be a useful tool to select these patients for additional prophylactic measures.
In patients with gynecologic cancer, the risk of VTE persists during adjuvant chemotherapy following surgery. One fifth of the VTE in our study occurred during chemotherapy treatment and all patients were in the intermediate/high‐risk group, with the majority scoring as high risk. The Thrombogyn score was not designed to determine the risk of thrombosis in ambulatory patients undergoing chemotherapy and larger prospective studies are required to investigate whether the Thrombogyn score is valid in this setting.
4.1. Limitations
Our study has certain limitations. Although there were significant differences between the derivation and validation cohorts in our study, the Thrombogyn score was derived from a single center and requires external validation. The Thrombogyn score was derived from a retrospective study and is limited by the accuracy of documentation from hospital and general practitioner records; the score is validated, however, in a prospectively collected cohort that mirrored the findings of the derivation cohort.
Our multivariable analysis did not meet the rule of thumb of 10 events per predictor, and hence our results have to be interpreted with caution; however, the derived score contains only 3 predictors and was validated in a separate cohort, which provides confidence in the derivation model.
Histologic subtypes such as clear cell cancer, which are associated with a high rate of VTE, are underrepresented in the study, as they frequently present with VTE prior to cancer diagnosis and hence would be excluded. Similarly, patients who developed VTE during neoadjuvant chemotherapy are not captured in this study.
The thrombin‐generation assay is not routinely available; however, a recent study has shown that, when normalized, the thrombin‐generation assay has similar reproducibility to many standard coagulation assays used in the clinic. 43 Near‐patient testing has been described for the thrombin‐generation assay; hence, it has the potential to become a more accessible assay in the future. 44
Due to the lack of available plasma, we did not include the biomarkers in the original derivation model, and our biomarkers were selected based on previous evidence. 39 , 40 We cannot exclude the possibility that had they been included in the derivation model, they may not have been shown to be independent predictors. However, a large predictive study of a mixed cancer population has shown that D‐dimer is an independent predictor of VTE in a mixed cancer population. 26 The extended Thrombogyn score would require extensive validation before implementation could be considered.
Our follow‐up period was 24 months, and we acknowledge that the effects of additional hospitalizations and disease recurrence may modify VTE risk over time. In addition, our biomarker values are based on a single sample; repeated sampling during the follow‐up period may have been more powerful. However, the majority of VTE events occurred early in the follow‐up period, and our score was derived from a multivariable model that did include the variables associated with initial treatment, surgery, and hospital stay.
A strength of our study is that the majority or our patients were treatment naïve and underwent similar documented LMWH prophylactic regimens. Both the derivation and validation cohort contained significant numbers of both early‐stage and advanced cancers reflective of real‐life gynecologic cancer populations.
5. CONCLUSION
In conclusion, we have developed and validated the Thrombogyn score, a risk score for VTE in gynecologic cancer, which can successfully identify both high‐risk and low‐risk groups. Addition of ETP and D‐dimer values as biomarkers improves the predictive power of the score. Although external validation and ultimately randomized trials are required before the Thrombogyn score can be used in practice, the Thrombogyn score may offer a method to tailor prophylaxis in patients with gynecologic cancer at high risk for VTE, while reducing the need for extended thromboprophylaxis following surgery in low‐risk patients.
RELATIONSHIP DISCLOSURE
All authors declare no competing interests in relation to this study. FAS, NG, and LAN have received unrestricted educational grants and grants for investigator‐initiated studies from Leo Pharma.
AUTHOR CONTRIBUTIONS
LAN designed the study, analyzed and interpreted the data, and drafted the manuscript. MPW carried out the biomarker analysis, interpreted the data, and drafted the manuscript. SAO, ZM, and NI participated in the acquisition and analysis of the data and reviewed the manuscript. AK designed and performed the statistical analysis, assisted the interpretation of the data, and reviewed the manuscript. NG and FAS assisted in the design of the study, analysis of the data, and the drafting of the manuscript. All authors approved the final version prior to submission.
Supporting information
Fig S1
Fig S2
Table S1
Table S2
Table S3
ACKNOWLEDGMENTS
The authors gratefully acknowledge the support of the Health Research Board of Ireland, who funded this project (HRA‐POR‐2013‐236), and the support of a dissemination grant from Leo Pharma.
Norris LA, Ward MP, O'Toole SA, et al. A risk score for prediction of venous thromboembolism in gynecologic cancer: The Thrombogyn score. Res Pract Thromb Haemost. 2020;4:848–859. 10.1002/rth2.12342
Feras Abu Saadeh and Noreen Gleeson contributed equally to the manuscript.
Handling Editor: Susan Kahn.
Contributor Information
Lucy A. Norris, Email: lnorris@tcd.ie, @LucyNORTCD.
Mark P. Ward, @Wardm6TCD.
Sharon A. O'Toole, @Shotoole81.
Zibi Marchocki, @zibi06479123.
Feras Abu Saadeh, @FerasAbuSaadeh2.
REFERENCES
- 1. Barber EL, Clarke‐Pearson DL. Prevention of venous thromboembolism in gynecologic oncology surgery. Gynecol Oncol. 2017;144:420–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Satoh T, Matsumoto K, Tanaka YO, Akiyama A, Nakao S, Sakurai M, et al. Incidence of venous thromboembolism before treatment in cervical cancer and the impact of management on venous thromboembolism after commencement of treatment. Thromb Res. 2013;131:e127–e132. [DOI] [PubMed] [Google Scholar]
- 3. Duska LR, Garrett L, Henretta M, Ferriss JS, Lee L, Horowitz N. When “never events” occur despite adherence to clinical guidelines: the case of venous thromboembolism in clear cell cancer of the ovary compared with other epithelial histologic subtypes. Gynecol Oncol. 2010;116:374–7. [DOI] [PubMed] [Google Scholar]
- 4. Martino MA, Borges E, Williamson E, Siegfried S, Cantor AB, Lancaster J, et al. Pulmonary embolism after major abdominal surgery in gynecologic oncology. Obstet Gynecol. 2006;107:666–71. [DOI] [PubMed] [Google Scholar]
- 5. Farge D, Frere C, Connors JM, Ay C, Khorana AA, Munoz A, et al. International clinical practice guidelines in the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019;20:e566–e581. [DOI] [PubMed] [Google Scholar]
- 6. Nelson G, Bakkum‐Gamez J, Kalogera E, Glaser G, Altman A, Meyer LA, et al. Guidelines for perioperative care in gynecologic/oncology: Enhanced Recovery After Surgery (ERAS) Society recommendations‐2019 update. Int J Gynecol Cancer. 2019;29:651–68. [DOI] [PubMed] [Google Scholar]
- 7. Carrier M, Altman AD, Blais N, Diamantouros A, McLeod D, Moodley U, et al. Extended thromboprophylaxis with low‐molecular weight heparin (LMWH) following abdominopelvic cancer surgery. Am J Surg. 2019;218:537–50. [DOI] [PubMed] [Google Scholar]
- 8. Agnelli G, Bolis G, Capussotti L, Scarpa RM, Tonelli F, Bonizzoni E, et al. A clinical outcome‐based prospective study on venous thromboembolism after cancer surgery: the @RISTOS project. Ann Surg. 2006;243:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wagner BE, Langstraat CL, McGree ME, Weaver AL, Sarangi S, Mokri B, et al. Beyond prophylaxis: extended risk of venous thromboembolism following primary debulking surgery for ovarian cancer. Gynecol Oncol. 2019;152:286–92. [DOI] [PubMed] [Google Scholar]
- 10. Bouchard‐Fortier G, Geerts WH, Covens A, Vicus D, Kupets R, Gien LT. Is venous thromboprophylaxis necessary in patients undergoing minimally invasive surgery for a gynecologic malignancy? Gynecol Oncol. 2014;134:228–32. [DOI] [PubMed] [Google Scholar]
- 11. Kim JS, Mills KA, Fehniger J, Liao C, Hurteau JA, Kirschner CV, et al. Venous thromboembolism in patients receiving extended pharmacological prophylaxis after robotic surgery for endometrial cancer. Int J Gynecol Cancer. 2017;27:1774–82. [DOI] [PubMed] [Google Scholar]
- 12. Kahr HS, Christiansen OB, Høgdall C, Grove A, Mortensen RN, Torp‐Pedersen C, et al. Endometrial cancer does not increase the 30 day risk of venous thromboembolism following hysterectomy compared with benign disease. A Danish National Cohort Study. Gynecol Oncol. 2019;155:112–8. [DOI] [PubMed] [Google Scholar]
- 13. Petch S, Norris L, O'Toole S, Gleeson N, Abu Saadeh F. Peri operative venous thromboembolism prophylaxis in gynaecological cancer patients. A survey of current practice. Thromb Res. 2016;145:126–8. [DOI] [PubMed] [Google Scholar]
- 14. Freeman AH, Barrie A, Lyon L, Littell RD, Garcia C, Conell C, et al. Venous thromboembolism following minimally invasive surgery among women with endometrial cancer. Gynecol Oncol. 2016;142:267–72. [DOI] [PubMed] [Google Scholar]
- 15. Schmeler KM, Wilson GL, Cain K, Munsell MF, Ramirez PT, Soliman PT, et al. Venous thromboembolism (VTE) rates following the implementation of extended duration prophylaxis for patients undergoing surgery for gynecologic malignancies. Gynecol Oncol. 2013;128:204–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pant A, Liu D, Schink J, Lurain J. Venous thromboembolism in advanced ovarian cancer patients undergoing frontline adjuvant chemotherapy. Int J Gynecol Cancer. 2014;24:997–1002. [DOI] [PubMed] [Google Scholar]
- 17. Khorana AA, Dalal M, Lin J, Connolly GC. Incidence and predictors of venous thromboembolism among ambulatory patients undergoing chemotherapy in the United States. Cancer. 2013;119:648–55. [DOI] [PubMed] [Google Scholar]
- 18. Carrier M, Abou‐Nassar K, Mallick R, Tagalakis V, Shivakumar S, Schattner A, et al. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2019;380:711–9. [DOI] [PubMed] [Google Scholar]
- 19. Khorana AA, Soff GA, Kakkar AK, Vadhan‐Raj S, Riess H, Wun T, et al. Rivaroxaban for thromboprophylaxis in high‐risk ambulatory patients with cancer. N Engl J Med. 2019;380:720–8. [DOI] [PubMed] [Google Scholar]
- 20. Agnelli G. Direct oral anticoagulants for thromboprophylaxis in ambulatory patients with cancer. N Engl J Med. 2019;380:781–3. [DOI] [PubMed] [Google Scholar]
- 21. Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy‐associated thrombosis. Blood. 2008;111:4902–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ay C, Dunkler D, Marosi C, Chiriac AL, Vormittag R, Simanek R, et al. Prediction of venous thromboembolism in cancer patients. Blood. 2010;116:5377–82. [DOI] [PubMed] [Google Scholar]
- 23. Verso M, Agnelli G, Barni S, Gasparini G, LaBianca R. A modified Khorana risk assessment score for venous thromboembolism in cancer patients receiving chemotherapy: the Protecht score. Intern Emerg Med. 2012;7:291–2. [DOI] [PubMed] [Google Scholar]
- 24. Gerotziafas GT, Taher A, Abdel‐Razeq H, AboElnazar E, Spyropoulos AC, El Shemmari S. A predictive score for thrombosis associated with breast, colorectal, lung, or ovarian cancer. The prospective COMPASS‐Cancer Associated Thrombosis study. Oncologist. 2017;22:1222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Syrigos K, Grapsa D, Sangare R, Evmorfiadis I, Larsen AK, Van Dreden P, et al. Prospective assessment of clinical risk factors and biomarkers of hypercoagulability for the identification of patients with lung adenocarcinoma at risk for cancer‐associated thrombosis: The Observational ROADMAP‐CAT Study. Oncologist. 2018;23:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pabinger I, van Es N, Heinze G, Posch F, Riedl J, Reitter EM, et al. A clinical prediction model for cancer‐associated venous thromboembolism: a development and validation study in two independent prospective cohorts. Lancet Haematol. 2018;5:e289–e298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mansfield AS, Tafur AJ, Wang CE, Kourelis TV, Wysokinska EM, Yang P. Predictors of active cancer thromboembolic outcomes: validation of the Khorana score among patients with lung cancer. J Thromb Haemost. 2016;14:1773–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kuderer NM, Poniewierski MS, Culakova E, Lyman GH, Khorana AA, Pabinger I, et al. Predictors of venous thromboembolism and early mortality in lung cancer: results from a global prospective study (CANTARISK). Oncologist. 2018;23:247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87. [DOI] [PubMed] [Google Scholar]
- 30. Mutch DG. The new FIGO staging system for cancers of the vulva, cervix, endometrium and sarcomas. Gynecol Oncol. 2009;115:325–8. [Google Scholar]
- 31. Prat J, FIGO Committee on Gynecologic Oncology . Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet. 2014;124:1–5. [DOI] [PubMed] [Google Scholar]
- 32. Aletti GD, Dowdy SC, Podratz KC, Cliby WA. Relationship between surgical complexity, short term morbidity and overall survival in primary surgery for advanced ovarian cancer. Am J Obstet Gynecol. 2005;99:119–25. [DOI] [PubMed] [Google Scholar]
- 33. Abu Saadeh F, Langhe R, Galvin DM, O Toole SA, O'Donnell DM, Gleeson N, et al. Procoagulant activity in gynaecological cancer patients; the effect of surgery and chemotherapy. Thomb Res. 2016;139:135–41. [DOI] [PubMed] [Google Scholar]
- 34. Barber EL, Clarke‐Pearson DL. The limited utility of currently available venous thromboembolism risk assessment tools in gynecological oncology patients. Am J Obstet Gynecol. 2016;215(445):e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stroud W, Whitworth JM, Miklic M, Schneider KE, Finan MA, Scalici J, et al. Validation of a venous thromboembolism risk assessment model in gynecologic oncology. Gynecol Oncol. 2014;134:160–3. [DOI] [PubMed] [Google Scholar]
- 36. Cella CA, Di Minno G, Carlomagno C, Arcopinto M, Cerbone AM, Matano E, et al. Preventing venous thromboembolism in ambulatory cancer patients: the ONKOTEV study. Oncologist. 2017;22:601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Heit JA. Epidemiology of venous thrombosis. Nat Rev Cardiol. 2015;12:464–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Amirkhosravi A, Bigsby G 4th, Desai H, Rivera‐Amaya M, Coll E, Robles‐Carrillo L, et al. Blood clotting activation analysis for preoperative differentiation of benign versus malignant ovarian masses. Blood Coagul Fibrinolysis. 2013;24:510–7. [DOI] [PubMed] [Google Scholar]
- 39. Ward MP, Abu Saadeh F, O'Toole S, Marchocki Z, Gleeson N, Norris LA. Optimisation of thrombin generation as a predictive biomarker for venous thromboembolism in gynaecological malignancies. Res Pract Thromb and Haemost. 2018;2(suppl 1):PB518. [Google Scholar]
- 40. Ay C, Dunkler D, Simanek R, Thaler J, Koder S, Marosi C, et al. Prediction of venous thromboembolism in patients with cancer by measuring thrombin generation: results from the Vienna Cancer and Thrombosis study. J Clin Oncol. 2011;29:2099–103. [DOI] [PubMed] [Google Scholar]
- 41. Tripodi A. Thrombin generation assay and its application in the clinical laboratory. Clin Chem. 2016;62:699–707. [DOI] [PubMed] [Google Scholar]
- 42. van Veen JJ, Gatt A, Makris M. Thrombin generation testing in routine clinical practice: are we there yet? Br J Haematol. 2008;142:889–903. [DOI] [PubMed] [Google Scholar]
- 43. Ljungkvist M, Strandberg K, Berntorp E, Chaireti R, Holme PA, Larsen OH, et al. Evaluation of a standardized protocol for thrombin generation using the calibrated automated thrombogram: a Nordic study. Haemophilia. 2019;25:334–42. [DOI] [PubMed] [Google Scholar]
- 44. Moorlag M, Schurgers E, Krishnamoorthy G, Anne Bouwhuis A, Lindhout T, Kelchtermans HL. Near‐patient thrombin generation in patients undergoing elective cardiac surgery. J Appl Lab Med. 2017;6(1):613–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Table S1
Table S2
Table S3


