Abstract
Venous thromboembolism (VTE) is a major cause of morbidity and mortality. The impact of the Surgeon General’s Call to Action in 2008 has been lower than expected given the public health impact of this disease. This scientific statement highlights future research priorities in VTE, developed by experts and a crowdsourcing survey across 16 scientific organizations. At the fundamental research level (T0), researchers need to identify pathobiologic causative mechanisms for the 50% of patients with unprovoked VTE and better understand mechanisms that differentiate hemostasis from thrombosis. At the human level (T1), new methods for diagnosing, treating, and preventing VTE will allow tailoring of diagnostic and therapeutic approaches to individuals. At the patient level (T2), research efforts are required to understand how foundational evidence impacts care of patients (eg, biomarkers). New treatments, such as catheter‐based therapies, require further testing to identify which patients are most likely to experience benefit. At the practice level (T3), translating evidence into practice remains challenging. Areas of overuse and underuse will require evidence‐based tools to improve care delivery. At the community and population level (T4), public awareness campaigns need thorough impact assessment. Large population‐based cohort studies can elucidate the biologic and environmental underpinings of VTE and its complications. To achieve these goals, funding agencies and training programs must support a new generation of scientists and clinicians who work in multidisciplinary teams to solve the pressing public health problem of VTE.
Keywords: hemostasis , postthrombotic syndrome , pulmonary embolism, thrombosis, venous thromboembolism, venous thrombosis , research priorities
Essentials.
The article presents future research priorities in venous thromboembolism.
It was developed by experts and a crowdsourcing survey across 16 scientific organizations.
It covers fundamental (T0), human‐level (T1), patient‐level (T2), practice‐level (T3), and community‐ and population‐level (T4) research.
The authors suggest that multidisciplinary team science approaches be prioritized.
1. INTRODUCTION
Venous thromboembolism (VTE) remains a major cause of morbidity and mortality, affecting up to 1 million Americans and more than 700 000 Europeans annually. 1 Composed of both deep vein thrombosis (DVT) and pulmonary embolism (PE), VTE disproportionately impacts older adults worldwide. 2 An estimated 1‐in‐12 people will develop VTE after age 45. 3 Thirty‐day mortality is as high as 30% for patients with PE. 4 Emerging knowledge suggests that impaired quality of life is common. Up to 50% of patients with DVT will develop postthrombotic syndrome (PTS), which consists of pain, swelling, skin changes, and ulceration; 5%‐10% will have severe morbidity with reduced quality of life. 5
In 2008, the US surgeon general issued a call to action to prevent DVT and PE. 6 That document highlighted the unique opportunity for multiple stakeholders to coordinate efforts aimed at (i) increasing public awareness, (ii) supporting development of evidence‐based practices, and (iii) carrying out research to address gaps in knowledge. It is unclear how much progress has been made in the decade since that call to action. While some organizations champion patient, provider, and public awareness, efforts in translational and transformative research are not commensurate with the public health impact of VTE. 7
This statement outlines key research priorities to address knowledge gaps in VTE (Table 1). As outlined in Supplements S1 and S2, in 2018, members of 16 international organizations, including lead organizations for this project (American Heart Association, American Venous Forum, and ISTH) were invited in a crowdsourcing activity to share their priorities for VTE research through a survey. While attempts were made to include a global perspective, we did not collect participant location, and North American participation may be overrepresented. Informed by these results, invited experts presented their vision at the 2018 American Heart Association Vascular Discovery conference (San Francisco, CA), and the audience provided input. At that meeting, a writing group was formed to develop this scientific statement based on survey results. The final manuscript outlines key areas for future research across the spectrum of translational research (bench‐to‐bedside‐to‐population; Figure 1). As this article was going to production, the rapid realization of a new coagulopathy with marked VTE risk related to coronavirus disease 2019 has led to pressing need for basic, translational, and clinical research, including on antithrombotic treatments in these patients.
TABLE 1.
Some research priorities in venous thromboembolism across the spectrum of translational research
| T0 – Fundamental and discovery‐based research |
|
| T1 ‐ Human level research |
|
| T2 ‐ Patient level research |
|
| T3 ‐ Practice level research |
|
| T4 ‐ Community and population level research |
|
FIGURE 1.
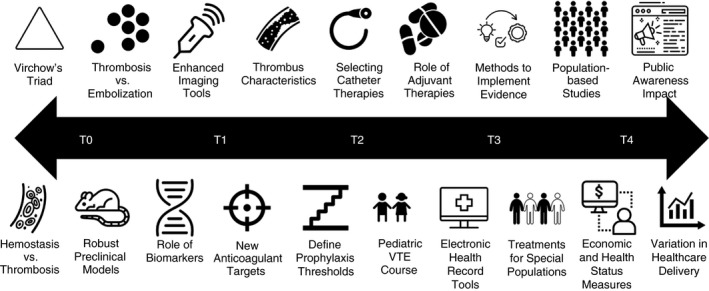
Priorities for future VTEresearch across the translational spectrum. VTE, venous thromboembolism; T0 indicates fundamental and discovery‐based research; T1, human level research; T2, patient level research; T3, practice level research; T4, community and population level research
2. T0 – FUNDAMENTAL RESEARCH: FROM MOLECULES TO BIOLOGICAL SYSTEMS
Most of the time, the coagulation system remains well balanced to respond to vascular injury without clotting within the vessels (hemostasis). However, when clot formation does occur within blood vessels (thrombosis), the effects are life threatening. Mechanisms that differentiate clot formation occurring in the setting of hemostasis versus those that promote thrombosis remain poorly understood. The fact that up to half of VTE cases lack an identifiable provoking trigger highlights a critical knowledge gap regarding the mechanisms that drive pathological thrombus formation.
A persistent gap in developing new approaches to treat and prevent VTE is inadequate understanding of the underlying pathophysiology. Virchow's triad of abnormalities in blood components, the vessel wall, and blood flow defines our understanding of thrombotic risk and provides a platform for fundamental and discovery‐based research into the mechanisms driving VTE. Researchers have largely taken a deconstructive approach focused on each component in isolation to determine its independent contribution to thrombus formation. Although these studies have defined numerous mechanisms regarding blood components and their role in VTE, 8 effects of vascular wall dysfunction and blood flow on physiological and pathological clot formation are still not well characterized. For example, genetic, biochemical, and animal studies of plasma clotting factors have robustly associated abnormal levels of certain plasma proteins with VTE risk. 9 However, the fact that many patients with these abnormalities do not develop VTE indicates that additional, coexisting abnormalities of thrombosis, vessel wall dysfunction, or environmental factors are necessary to promote thrombosis. Understanding the complex interactions within VTE risk factors is a driving need in VTE research.
In VTE, as in any thrombotic disease, pathological cross‐talk between the vessel wall and blood components is considered a driver of thrombosis. This complex scenario is difficult to reproduce in a laboratory setting. Over the years, the scientific community has recognized the importance of both in vitro (eg, cell coculture, microfluidic, and computational models) and in vivo (eg, vena cava ligation, FeCl3 injury) preclinical models to understand thrombosis and evaluate potential treatments. All current VTE preclinical models have pros and cons. Understanding these strengths and limitations is imperative when choosing models in the context of a given research question.
Given the strength that in vivo models can simultaneously incorporate all 3 arms of Virchow’s triad, animal research has become an essential tool in efforts to define pathophysiologic mechanisms in VTE and has significantly advanced understanding of cellular and biochemical mechanisms. However, live models have their limitations based on species, size, and life span. These differences can limit their application to the human experience of VTE. For example, most PE models do not replicate the human experience where a DVT embolizes from the deep veins to the lungs. Instead, they commonly rely on protein infusion locally to incite thrombosis. Developing new models that more closely mimic human pathobiology (including embolism) is a high priority given PE‐related mortality and differences in DVT‐ and PE‐specific risk factors. 10
3. T1 – TRANSLATIONAL RESEARCH: FROM ANIMALS TO HUMANS
Significant advances in diagnosing, treating, and preventing VTE depends on translating fundamental and discovery‐based research findings to humans (T1 research). A high priority in diagnosis of VTE is elucidating thrombus chronicity or embolic potential with imaging that incorporates information on thrombus pathophysiology. This might improve diagnostic accuracy and influence treatment decisions. For example, a lower‐extremity thrombus with imaging characteristics that suggest low embolic potential may be safely treated with shorter courses of anticoagulation, while one with higher embolic potential may warrant longer courses of anticoagulation or the placement of an inferior vena cava filter if anticoagulation is contraindicated.
The emergence of direct oral anticoagulants (DOACs) has transformed VTE treatment; however, the search continues for even safer treatments. 11 Recent epidemiological studies and animal models show relationships between a number of cloting factors (eg, factors XIa, XII, and IX) and VTE suscepibility. For example, factor XIa inhibition is emerging as a promising therapeutic strategy with the potential of limited bleeding complications. 12 While various factor inhibitor agents move through the clinical trials pipeline, carefully designed studies should concurrently identify optimal treatment strategies based on patient and VTE charactersitics.
Independent of therapy choice, identifying patients at greatest risk for recurrent VTE, who might benefit from long‐term secondary prevention, remains a challenge. 6 Research on defining treatment duration that extends beyond consideration of presenting characteristics (eg, provoked versus unprovoked VTE) is warranted. Significant progress in this area may be possible using innovative imaging and biomarker assessments. Biomarkers other than D‐dimer that predict VTE recurrence risk are needed; candidates include soluble P‐selectin, factor VIII, factor IX, extracellular DNA, and intercellular adhesion molecule 1, but new biomarkers should be sought. 13 , 14 High‐resolution imaging and proteomic analysis of thrombi may provide new mechanistic biomarkers of recurrence. 15 Other avenues to pursue include genetic screening, which is complicated by epigenetic factors that also contribute to disease. 16 Unbiased “omics” approaches that measure circulating microRNAs has identified candidates that are associated with VTE recurrence. 17 Metabolic screening has also shown potential to identify new biomarkers that influence VTE. 18 , 19 In sum, personalized approaches to treatment that integrate thrombus pathophysiology, circulating biomarkers, patient characteristics, and patient preferences require study. 20
4. T2 – CLINICAL RESEARCH: FROM HUMANS TO PATIENTS
Clinicans struggle to translate findings from discovery‐based research to care of individual patients (T2 research). For example, selecting therapies based on VTE recurrence risk remains a largely unfulfilled goal. As noted above, the use of new biomarkers may offer “personalization” opportunities in VTE treatment. However, challenges remain in translating the findings from T0 and T1 research to large cohorts that can account for the heterogeneity in populations while assessing if specific therapies influence clot structure in a manner that impacts clinical outcome. 21
Catheter‐based therapies, including thrombolysis, are increasingly used for patients with acute PE and/or DVT. Determining patients most likely to benefit from an invasive procedure is needed. 22 At the same time, clinical, biomarker, and echocardiographic parameter collection (in PE) is necessary for prospective validation of many different risk stratification tools.
The impact of therapy on long‐term outcomes is not well described. For example, while pharmacomechanical thrombolysis may not prevent PTS after proximal DVT in general, efficacy in selected patients based on anatomic presentation and persistence of symptoms despite anticoagulation is unknown. The same is true for use of catheter‐based therapies in patients with intermediate‐ and high‐risk PE to prevent long‐term dyspnea and fatigue associated with the so‐called post‐PE syndrome.
Few modalities have demonstrated benefit in preventing PTS in patients with DVT. Specifically, compression stockings failed to prevent PTS in at least 1 large randomized study. 23 However, other treatments to prevent PTS merit study, including different anticoagulant strategies, P2Y12 inhibitors, adhesion molecule inhibitors, venoactive drugs, and statins. Finally, limited research is available on effective treatment of PTS, including the roles of the above medications and venous surgical interventions.
Optimizing VTE prevention in hospitalized medical and surgical patients can reduce the population burden of VTE. Several questions require research: identification of patients at highest risk of VTE and bleeding to guide prophylaxis type and duration; understanding why “breakthrough” VTE occurs in hospitalized patients receiving prophylaxis; identification of methods to enhance compliance with prophylaxis 24 , 25 ; and methods to reduce overuse of prophylaxis, which is both costly and potentially dangerous. Studies of deimplementation that reduce overuse of therapies (eg, VTE prophylaxis in low‐risk patients) are equally important.
Finally, management of VTE in pediatric and pregnant patients remains understudied. The incidence of VTE in pediatric patients is low. 26 Harnessing a multicenter consortium to pool standardized anatomic, therapeutic, and demographic data with long‐term follow‐up may further define the clinical course of VTE in children. VTE in pregnancy is a highly morbid complication. While low‐molecular‐weight heparin is standard of care for prophylaxis in high‐risk women, major gaps remain in assessing the absolute VTE risk, selecting dose, and determining duration of prophylaxis, 27 , 28 and in optimal treatment when VTE occurs in pregnant women.
5. T3 – TRANSLATIONAL RESEARCH: FROM PATIENTS TO CLINICAL PRACTICE
While large‐scale clinical trials can establish the efficacy of various interventions (both prophylactic and treatment), implementing these into clinical practice (T3 research) remains a barrier to improved health. Important aspects of evidence‐to‐practice translation are both the overuse and underuse of treatments. Examples of overuse include placement of inferior vena cava filters for primary prophylaxis in patients at risk for VTE and use of catheter‐directed thrombolysis for treating patients with intermediate‐ and high‐risk PE without randomized trial evidence supporting mortality benefits. 29 , 30 , 31 Examples of underuse include differential DOAC prescribing and low use of outpatient DVT treatment based on race and socioeconomic factors. 32 , 33 Tools (eg, prediction models) are needed to help clinicians select patients most likely to benefit from specific interventions. Integrating these into the electronic medical record may improve safe medication delivery. Additionally, identification of strategies aimed at changing clinician behavior to adopt evidence‐based practices are critically important.
The rapid growth in use of devices (eg, vena cava filters, venous stents, thrombolysis, and thrombectomy catheters) to treat patients with VTE presents a clinical dilemma. Devices often achieve regulatory approval based largely on safety profile, while randomized controlled trials comparing these devices to noninterventional approaches and between different devices are needed to determine clinical efficacy. Also, postmarketing assessment of device utilization, efficacy, and safety is needed. Well‐designed population‐based registries can play a role in determining the profile of patients in which these devices are being used, what clinical benefits can be expected, which patients are more likely to experience benefit, and what risks are associated with use of the devices outside of research settings.
While each of the DOACs have undergone large‐scale trials, some populations were inadequately represented, and race/ethnicity of trial participants was not always diverse. Notable examples of other under‐represented groups include patients with severe renal impairment or who are receiving hemodialysis, those at extremes of weight, those with reduced absorption due to gastrointestinal surgery, those with autoimmune diseases, and those who have had venous stenting procedures. 34 , 35 High‐quality efficacy and safety data for DOAC use in cerebral and portal venous thrombosis is also lacking. Since it is impractical to conduct randomized trials in each of these patient groups, observational studies are needed to further assess safety and efficacy.
Finally, many inherited and acquired thrombophilias can be diagnosed in patients with VTE, and some increase risk of recurrence after a first event. However, only D‐dimer has been adequately studied for guiding management decisions, and little is known on the overall health impact and economics of thrombophilia testing, in both patients and their relatives. More work is needed to understand the benefits and harms of genetic and nongenetic thrombophilia testing and how best to integrate that information into management and prevention.
Across a range of treatment modalities, better equipping physicians and health care systems to translate evidence into practice is needed. This includes identifying subpopulations most likely to benefit from therapies, exploring therapeutic benefits in populations not typically included in randomized trials, and understanding the impact of diagnostic testing on care at the practice and population levels for patients with VTE.
6. T4 – GLOBAL RESEARCH: FROM CLINICAL PRACTICE TO HEALTHCARE SYSTEMS
Public awareness and public health efforts to address VTE prevention and treatment have a limited evidence base (T4 research). Despite VTE being a common disease, few in the public are aware of its signs, symptoms, and risk factors. 36 Campaigns such as World Thrombosis Day, initiatives from the American Heart Association, and other efforts may increase awareness, but more studies are needed to gauge improvement in public knowledge based on these programs.
Analogous to research efforts in atherosclerosis, large population‐based epidemiology studies are needed to better understand the biologic and environmental causes of VTE, and the range of nonthrombotic outcomes in patients who have experienced VTE (described in Supplements S1 and S2). Data from these studies could generate hypotheses on causal mechanisms of VTE and be harnessed to design clinical trials of preventive and therapeutic treatments that precisely target genetic, molecular, clinical, and/or environmental mechanisms associated with VTE and its recurrence. These studies would include collection of blood and tissue samples for storage in biorepositories for subsequent analysis. Information ranging from genomics, transcriptomics, proteomics, and metabolomics would be integrated with demographic, clinical, laboratory, imaging information, and exposures (including socioeconomic and other environmental characteristics) to create large databases that could be shared. Outcomes after VTE for conditions that share risk factors with VTE (eg, kidney disease) and psychosocial outcomes after VTE (eg, depression) are poorly understood.
Up to 50% of patients develop long‐term exercise limitation after PE or the PTS following DVT. 37 , 38 Yet the effect of DVTs and PEs on long‐term health status and societal impacts for many of those afflicted is not well established. More population‐based studies are needed to examine patient‐centered outcomes, including long‐term symptoms, functional status, and consequent effects on quality of life. 39 These studies should use or develop disease‐specific measures whenever possible. 37 , 40 , 41 , 42 Furthermore, studies are needed to determine best methods for integrating traditional methods of collecting patient‐reported quality of life outcomes along with digital health tools, such as wearable sensors, smartphones, and point‐of‐care devices that monitor biometrics. 43 , 44
Population‐based studies are needed to determine the effect of health care delivery on VTE outcomes. These include comparative effectiveness studies assessing clinical and economic end points and studies addressing implementation of evidence‐based practices (eg, VTE risk assessment and prophylaxis in hospitalized patients). Given the well‐documented disparities in health and health care in minority populations in the United States, the latter related to access and outcomes, special attention should be afforded to those populations to address specific predilections and outcomes in those with VTE. This research could involve analysis of data from electronic health records, observational registries, and insurance and administrative claims databases, which would enable assessment of how nonclinical factors like education, income, insurance coverage and payment policies, and governmental regulations influence diffusion and uptake of effective therapies to affect mortality and morbidity from VTE, and the quality of life of patients affected by VTE. 45 There is an unmet need to define and study similar disparities in other countries.
7. IMPORTANCE OF INTERDISCIPLINARY APPROACHES
The VTE research field needs answers, and the answers cannot come from one single research tool. Collaboration among experts in each preclinical and clinical area will provide optimal insight to the field and to the patients, the ultimately beneficiary of our daily efforts.
We propose multidisciplinary approaches that integrate epidemiologic, genomic, cellular, biochemical, and biophysical strategies to advance fundamental understanding and translate knowledge to patient care.
Practical methods to study multiple risk factors in concert lag, in part from the complexity of investigations involving multidisciplinary concepts. These studies often require harmonization of complicated and field‐specific language to describe technically challenging methods and detailed findings. However, efforts to bridge these gaps and strengthen collaborations are likely to yield new information on pathophysiologic mechanisms. For example, a recent approach to combine in vivo and in vitro analyses with computational modeling and bioengineered microfluidic chambers revealed effects of elevated hematocrit on platelet accumulation within thrombi that were not appreciable in mouse models alone, demonstrating the power of interdisciplinary collaborations. 46 Accordingly, additional multidisciplinary studies to elucidate mechanisms in VTE are warranted. Devices permiting control of fluid mechanics may enable more controlled studies of the contribution of blood flow than is possible in mice. Studies using biologically engineered “blood vessels” with innovative designs may expose vascular responses to changes in flow, as well as interactions between blood cells and proteins with the vessel wall during DVT. 47 Similarly, integrating approaches in genomics and epidemiology with functional analysis of molecular mechanisms may define additional pathways that contribute to VTE. This kind of integrated approach may alleviate confounding “noise” in genetic analysis and provide specific and focused hypotheses to guide biological and biochemical studies in new directions. Pathways identified and characterized through these collaborations may provide robust new therapeutic targets and translate genetic discovery to practical applications in the clinic. Facilitating multidisciplinary science teams via specific funding mechanisms is a major priority for advancing in VTE research.
8. BARRIERS AND OPPORTUNITIES
To solve the problems outlined above, we need to bring together scientists and clinicians from disparate disciplines, including those not traditionally involved in VTE research. For example, at the intersection of rehabilitation science, epidemiology, clinical investigation, health services research, and big data sits an opportunity to explore the prevalence, impact, and potential therapies of the post‐PE syndrome.
While progress is being made in prevention and treatment of cancer‐associated VTE, many questions across the translational spectrum remain. These include mechanistic, preventative, and therapeutic questions about this high‐mortality condition. Multidisciplinary teams may employ different approaches to better understand the etiology, prevention, and treatment of cancer‐associated VTE as a distinct entity from non–cancer‐associated VTE.
The broad adoption of electronic health records presents an opportunity to gather large quantities of data for retrospective analysis and to screen for patient enrollment in research studies. However, without improvements in quality and availability of natural language processing in electronic health records, much of the data stored is not easily searchable, presenting a major barrier to innovation. Additionally, challenges with interoperability between health systems and electronic health record platforms stifles potential large‐scale studies and collaborative efforts.
9. CONCLUSION
As a leading cause of death and disability, efforts to improve the prevention, diagnosis, and management of VTE are vitally important. Across the spectrum of translational research, opportunities exist to transform the care of patients with VTE. New scientists who become invigorated to explore these high‐need areas will have a tremenous impact on the population’s health. It is imperative that funding agencies and training programs support the next generation of scientists who will solve many of these pressing public health problems.
RELATIONSHIP DISCLOSURE
Drs. Cushman, Creager, Diaz, Henke, Machlus and Nieman report no relevant disclosures. Dr. Barnes discloses grant funding from Blue Cross, Blue Shield of Michigan and Pfizer/Bristol‐Myers Squibb, and is on consultant or advisory boards for AMAG Pharmaceuticals, Janssen, Pfizer/Bristol‐Myers Squibb, and Portola. Dr. Wolberg reports research funding from Novo Nordisk, BMS, and Shire, and consulting for Bio Products Laboratory.
Supporting information
Supplement S1
Supplement S2
Cushman M, Barnes GD, Creager MA, et al. Venous thromboembolism research priorities: A scientific statement from the American Heart Association and the International Society on Thrombosis and Haemostasis. Res Pract Thromb Haemost. 2020;4:714–721. 10.1002/rth2.12373
Endorsed by the American Venous Forum
Contributor Information
Mary Cushman, Email: mary.cushman@uvm.edu, @MaryCushman.
Geoffrey D. Barnes, @GBarnesMD.
Mark A. Creager, @DrMarkCreager.
Jose A. Diaz, @JoseDiazVUMC.
Peter K. Henke, @Henke1965.
Kellie R. Machlus, @theClotThickens.
Marvin T. Nieman, @MarvNieman.
Alisa S. Wolberg, @ASWolberg.
REFERENCES
- 1. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics‐2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 2. ISTH Steering Committee for World Thrombosis Day . Thrombosis: a major contributor to the global disease burden. J Thromb Haemost. 2014;12:1580–90. [DOI] [PubMed] [Google Scholar]
- 3. Bell EJ, Lutsey PL, Basu S, Cushman M, Heckbert SR, Lloyd‐Jones DM, et al. Lifetime risk of venous thromboembolism in two cohort studies. Am J Med. 2016;129:339.e19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sogaard KK, Schmidt M, Pedersen L, Horvath‐Puho E, Sorensen HT. 30‐year mortality after venous thromboembolism: a population‐based cohort study. Circulation. 2014;130:829–36. [DOI] [PubMed] [Google Scholar]
- 5. Rabinovich A, Kahn SR. The postthrombotic syndrome: current evidence and future challenges. J Thromb Haemost. 2017;15:230–41. [DOI] [PubMed] [Google Scholar]
- 6. Office of the Surgeon General (US) . The Surgeon General’s call to action to prevent deep vein thrombosis and pulmonary embolism. Rockville, MD: Office of the Surgeon General (US); 2008. [PubMed] [Google Scholar]
- 7. American Heart Association . Improving vascular disease prevention, detection and treatment. [Accessed 2019 July 22] Available from https://professional.heart.org/idc/groups/ahamah‐public/@wcm/@sop/@scon/documents/downloadable/ucm_478975.pdf
- 8. Wolberg AS, Aleman MM, Leiderman K, Machlus KR. Procoagulant activity in hemostasis and thrombosis: Virchow’s triad revisited. Anesth Analg. 2012;114:275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wolberg AS, Rosendaal FR, Weitz JI, Jaffer IH, Agnelli G, Baglin T, et al. Venous thrombosis. Nat Rev Dis Pri. 2015;1:15006. [DOI] [PubMed] [Google Scholar]
- 10. van Langevelde K, Flinterman LE, van Hylckama VA, Rosendaal FR, Cannegieter SC. Broadening the factor V Leiden paradox: pulmonary embolism and deep‐vein thrombosis as 2 sides of the spectrum. Blood. 2012;120:933–46. [DOI] [PubMed] [Google Scholar]
- 11. Weitz JI, Fredenburgh JC. Factors XI and XII as targets for new anticoagulants. Front Med. 2017;4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Quan ML, Pinto DJP, Smallheer JM, Ewing WR, Rossi KA, Luettgen JM, et al. Factor XIa inhibitors as new anticoagulants. J Med Chem. 2018;61:7425–47. [DOI] [PubMed] [Google Scholar]
- 13. Jacobs B, Obi A, Wakefield T. Diagnostic biomarkers in venous thromboembolic disease. J Vasc Surg Venous Lymphat Disord. 2016;4:508–17. [DOI] [PubMed] [Google Scholar]
- 14. Rabinovich A, Cohen JM, Prandoni P, Kahn SR. Association between thrombophilia and the post‐thrombotic syndrome: a systematic review and meta‐analysis. J Thromb Haemost. 2014;12:14–23. [DOI] [PubMed] [Google Scholar]
- 15. Stachowicz A, Siudut J, Suski M, Olszanecki R, Korbut R, Undas A, et al. Optimization of quantitative proteomic analysis of clots generated from plasma of patients with venous thromboembolism. Clin Proteomics. 2017;14:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Benincasa G, Costa D, Infante T, Lucchese R, Donatelli F, Napoli C. Interplay between genetics and epigenetics in modulating the risk of venous thromboembolism: a new challenge for personalized therapy. Thromb Res. 2019;177:145–53. [DOI] [PubMed] [Google Scholar]
- 17. Wang X, Sundquist K, Svensson PJ, Rastkhani H, Palmer K, Memon AA, et al. Association of recurrent venous thromboembolism and circulating microRNAs. Clin Epigenetics. 2019;11:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goldenberg NA, Everett AD, Graham D, Bernard TJ, Nowak‐Gottl U. Proteomic and other mass spectrometry based “omics” biomarker discovery and validation in pediatric venous thromboembolism and arterial ischemic stroke: current state, unmet needs, and future directions. Proteomics Clin Appl. 2014;8:828–36. [DOI] [PubMed] [Google Scholar]
- 19. Deguchi H, Banerjee Y, Trauger S, Siuzdak G, Kalisiak E, Fernandez JA, et al. Acylcarnitines are anticoagulants that inhibit factor Xa and are reduced in venous thrombosis, based on metabolomics data. Blood. 2015;126:1595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wolberg AS, Mackman N. Venous thromboembolism: risk factors, biomarkers, and treatment. Arterioscler Thromb Vasc Biol. 2009;29:296–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Janion‐Sadowska A, Natorska J, Siudut J, Zabczyk M, Stanisz A, Undas A. Plasma fibrin clot properties in the G20210A prothrombin mutation carriers following venous thromboembolism: the effect of rivaroxaban. Thromb Haemost. 2017;117:1739–49. [DOI] [PubMed] [Google Scholar]
- 22. Chatterjee S, Chakraborty A, Weinberg I, Kadakia M, Wilensky RL, Sardar P, et al. Thrombolysis for pulmonary embolism and risk of all‐cause mortality, major bleeding, and intracranial hemorrhage: a meta‐analysis. JAMA. 2014;311:2414–21. [DOI] [PubMed] [Google Scholar]
- 23. Kahn SR, Shapiro S, Wells PS, Rodger MA, Kovacs MJ, Anderson DR, et al. Compression stockings to prevent post‐thrombotic syndrome: a randomised placebo‐controlled trial. Lancet. 2014;383:880–8. [DOI] [PubMed] [Google Scholar]
- 24. Lau BD, Streiff MB, Pronovost PJ, Haut ER. Venous thromboembolism quality measures fail to accurately measure quality. Circulation. 2018;137:1278–84. [DOI] [PubMed] [Google Scholar]
- 25. Schunemann HJ, Cushman M, Burnett AE, Kahn SR, Beyer‐Westendorf J, Spencer FA, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2:3198–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thompson AJ, McSwain SD, Webb SA, Stroud MA, Streck CJ. Venous thromboembolism prophylaxis in the pediatric trauma population. J Pediatr Surg. 2013;48:1413–21. [DOI] [PubMed] [Google Scholar]
- 27. Bates SM, Rajasekhar A, Middeldorp S, McLintock C, Rodger MA, James AH, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: venous thromboembolism in the context of pregnancy. Blood Adv. 2018;2:3317–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ward CM, Andrews RK. Illustrated state‐of‐the‐art capsules of the ISTH 2019 congress in Melbourne, Australia. Res Pract Thromb Haemost. 2019;3:431–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bikdeli B, Chatterjee S, Desai NR, Kirtane AJ, Desai MM, Bracken MB, et al. Inferior vena cava filters to prevent pulmonary embolism: systematic review and meta‐analysis. J Am Coll Cardiol. 2017;70:1587–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kucher N, Boekstegers P, Muller OJ, Kupatt C, Beyer‐Westendorf J, Heitzer T, et al. Randomized, controlled trial of ultrasound‐assisted catheter‐directed thrombolysis for acute intermediate‐risk pulmonary embolism. Circulation. 2014;129:479–86. [DOI] [PubMed] [Google Scholar]
- 31. Gayou EL, Makary MS, Hughes DR, Hemingway J, Elliott ED, Spain JW, et al. Nationwide trends in use of catheter‐directed therapy for treatment of pulmonary embolism in Medicare beneficiaries from 2004 to 2016. J Vasc Interv Radiol. 2019;30:801–6. [DOI] [PubMed] [Google Scholar]
- 32. Nathan AS, Geng Z, Dayoub EJ, Khatana SAM, Eberly LA, Kobayashi T, et al. Racial, ethnic, and socioeconomic inequities in the prescription of direct oral anticoagulants in patients with venous thromboembolism in the United States. Circ Cardiovasc Qual Outcomes. 2019;12:e005600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Douce D, McClure LA, Lutsey P, Cushman M, Zakai NA. Outpatient treatment of deep vein thrombosis in the United States: the reasons for geographic and racial differences in stroke study. J Hosp Med. 2017;12:826–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moll S, Crona DJ, Martin K. Direct oral anticoagulants in extremely obese patients: OK to use? Res Pract Thromb Haemost. 2019;3:152–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Padrnos LJ, Garcia D. May‐Thurner syndrome and thrombosis: a systematic review of antithrombotic use after endovascular stent placement. Res Pract Thromb Haemost. 2019;3:70–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wendelboe AM, McCumber M, Hylek EM, Buller H, Weitz JI, Raskob G, et al. Global public awareness of venous thromboembolism. J Thromb Haemost. 2015;13:1365–71. [DOI] [PubMed] [Google Scholar]
- 37. Kahn SR, Hirsch AM, Akaberi A, Hernandez P, Anderson DR, Wells PS, et al. Functional and exercise limitations after a first episode of pulmonary embolism: results of the ELOPE prospective cohort study. Chest. 2017;151:1058–68. [DOI] [PubMed] [Google Scholar]
- 38. Ten Cate‐Hoek AJ. Prevention and treatment of the post‐thrombotic syndrome. Res Pract Thromb Haemost. 2018;2:209–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rumsfeld JS, Alexander KP, Goff DC Jr, Graham MM, Ho PM, Masoudi FA, et al. Cardiovascular health: the importance of measuring patient‐reported health status: a scientific statement from the American Heart Association. Circulation. 2013;127:2233–49. [DOI] [PubMed] [Google Scholar]
- 40. Klok FA, Cohn DM, Middeldorp S, Scharloo M, Buller HR, van Kralingen KW, et al. Quality of life after pulmonary embolism: validation of the PEmb‐QoL Questionnaire. J Thromb Haemost. 2010;8:523–32. [DOI] [PubMed] [Google Scholar]
- 41. Kahn SR, Lamping DL, Ducruet T, Arsenault L, Miron MJ, Roussin A, et al. VEINES‐QOL/Sym questionnaire was a reliable and valid disease‐specific quality of life measure for deep venous thrombosis. J Clin Epidemiol. 2006;59:1049–56. [DOI] [PubMed] [Google Scholar]
- 42. Ghanima W, Wik HS, Tavoly M, Enden T, Jelsness‐Jorgensen LP. Late consequences of venous thromboembolism: measuring quality of life after deep vein thrombosis and pulmonary embolism. Thromb Res. 2018;164:170–6. [DOI] [PubMed] [Google Scholar]
- 43. McConnell MV, Shcherbina A, Pavlovic A, Homburger JR, Goldfeder RL, Waggot D, et al. Feasibility of obtaining measures of lifestyle from a smartphone app: the MyHeart Counts Cardiovascular Health Study. JAMA Cardiol. 2017;2:67–76. [DOI] [PubMed] [Google Scholar]
- 44. Topol EJ. A decade of digital medicine innovation. Sci Transl Med. 2019;11:eaaw7610. [DOI] [PubMed] [Google Scholar]
- 45. Maddox TM, Albert NM, Borden WB, Curtis LH, Ferguson TB Jr, Kao DP, et al. The learning healthcare system and cardiovascular care: a scientific statement from the American Heart Association. Circulation. 2017;135:e826–e857. [DOI] [PubMed] [Google Scholar]
- 46. Walton BL, Lehmann M, Skorczewski T, Holle LA, Beckman JD, Cribb JA, et al. Elevated hematocrit enhances platelet accumulation following vascular injury. Blood. 2017;129:2537–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tsai M, Kita A, Leach J, Rounsevell R, Huang JN, Moake J, et al. In vitro modeling of the microvascular occlusion and thrombosis that occur in hematologic diseases using microfluidic technology. J Clin Invest. 2012;122:408–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement S1
Supplement S2


