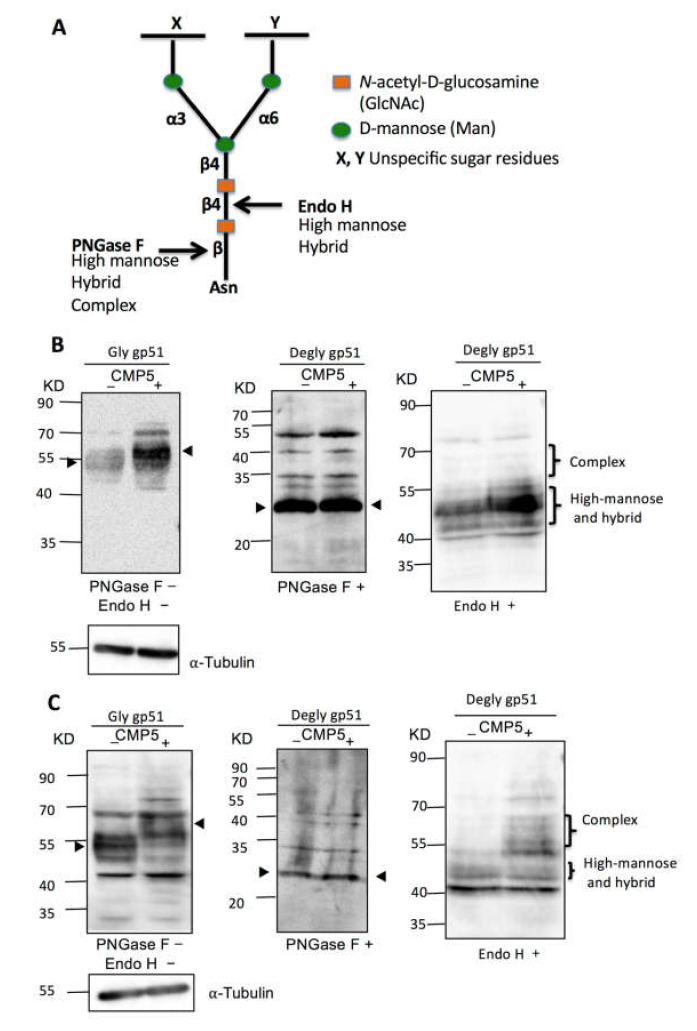
Figure 7.
Deglycosylation assay of BLV gp51 using PNGase F and endo H. (A) Schematic representation of PNGase F and Endo H sensitive bonds in the core of N-glycans. (B) FLK-BLV or (C) PK15-BLV were treated with Milli-Q water (CMP5−) or 20 µM CMP5 (CMP5+) for 48 h. Cell lysates were divided into three parts: one part was untreated (PNGase F−/Endo H−), the second part was deglycosylated by PNGase F (PNGase F+), and the third part was deglycosylated by Endo H (Endo H+). The cell lysates from the three conditions were subjected to Western blotting analysis with anti BLV gp51 (BLV2). α-Tubulin was used as a loading control. The data are a representation of at least three experiments. The positions of BLV gp51 protein with or without glycosylation, gp51 glycosylation pattern such as complex, and high-mannose and hybrid pattern, in addition to α-tubulin protein, are indicated.