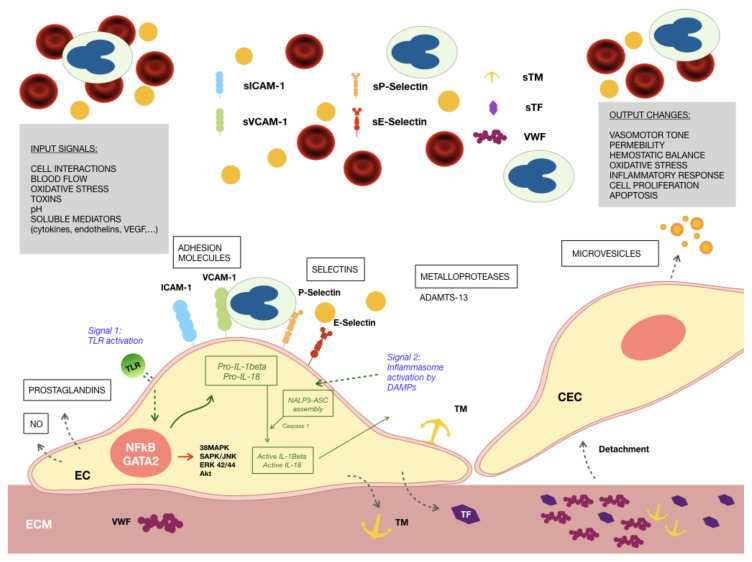
Figure 1.
Endothelial cells exposed to the uremic environment. Different factors alter the phenotype of endothelial cells (EC), showing signs of inflammation, oxidative stress and a prothrombotic behavior of the produced extracellular matrix (ECM): increased expression of adhesion molecules, such as Intercellular Adhersion Molecule 1 (ICAM-1), Vascular Cell Adhesion Molecule 1 (VCAM-1), and P- and E- selectins, both membrane-bound and soluble; thrombogenic proteins, such as von Willebrand Factor (VWF), tissue factor (TF) and thrombomodulin (TM) secreted to the ECM; production of reactive oxygen species (ROS) intracellularly. Activation of innate immunity mechanisms occurs in response to the presence of intracellular and soluble damage-associated molecular patterns (DAMPS). Signal 1: Toll-like receptor 4 (TLR4) is overexpressed in ECs exposed to the uremic milieu, being able to detect DAMPS leading to the production of the inactive forms of interleukins 1β and 18 (IL-1β and IL-18); Signal 2: DAMPS also promote the engagement of the inflammasome NOD-like receptor prying domain-containing-3 (NALP3), with the activation of IL-1β and IL-18. Activated nuclear factor kappa B (NFκB), linked to the inflammatory and oxidative stress responses of ECs to uremic media is related to increases in the degree of phosphorylation of ERK 42/44, SAPK/JNK, and AKT. These phenotypic alterations of ECs result in the recruitment of circulating leucocytes to the endothelial surface and, along with disrupted cell–cell contacts, their extravasation to the subendothelium to maintain the inflammatory response. Endothelial cells have a tendency to detach from their vascular bed passing to the circulation as circulating endothelial cells (CECs), and exposing an ECM highly reactive to circulating platelets.