Figure 8. RVM GABAergic Neurons Receive Inputs from Brain Structures Critical for Stress Responses and Differentially Engage Penk+ GABAergic Neurons in the Spinal Cord.
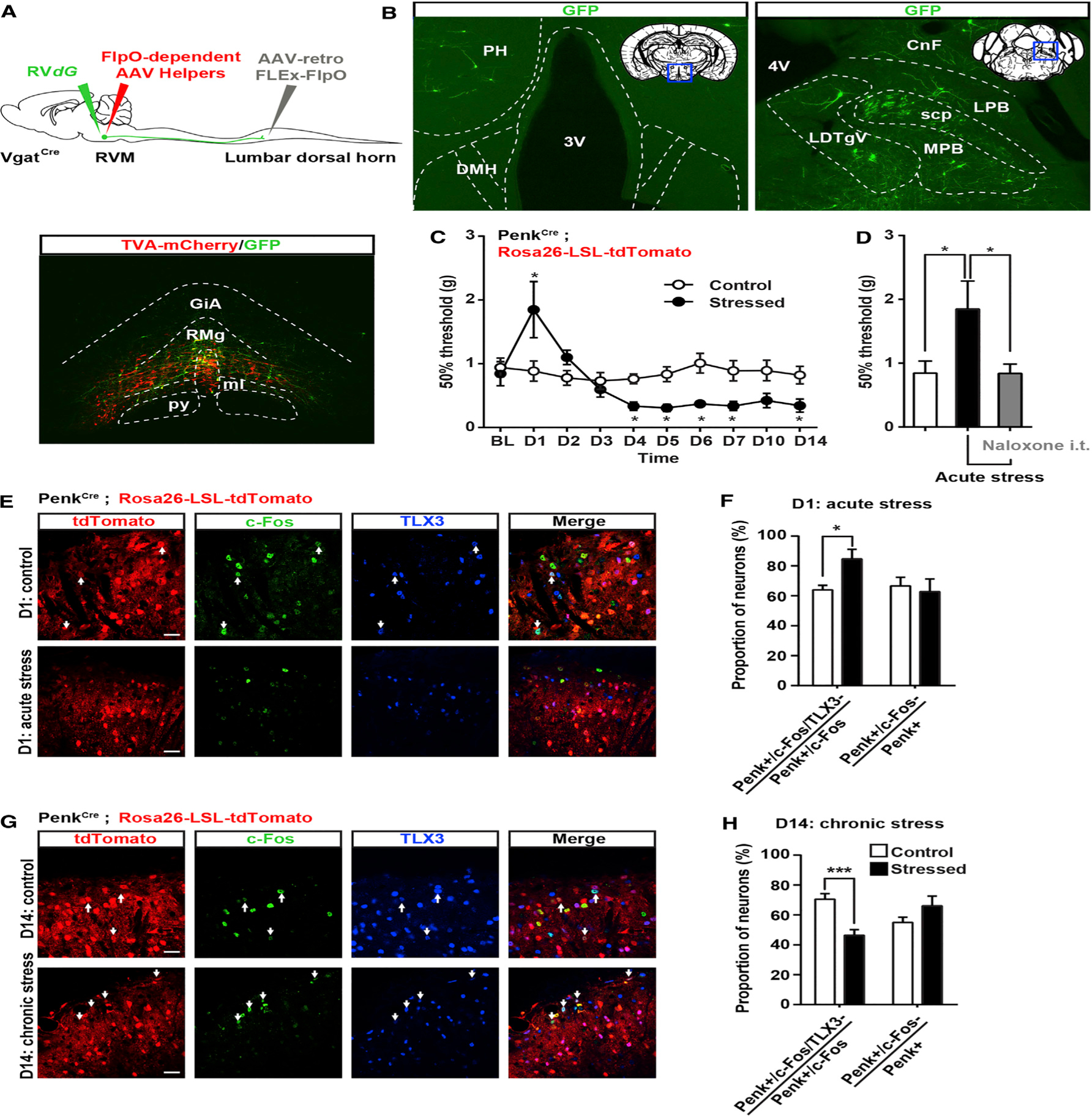
(A) Similar strategy as in Figures 2 and S4 to infect VgatCre neurons projecting to the spinal cord with AAV-retro-FLEx-FlpO in the spinal cord, flpO-dependent AAV helpers (red), and RVdG (green) in the RVM.
(B) Expression of GFP in the lateral hypothalamus (left) or lateral parabrachial nucleus (right) reveals that RVM GABAergic neurons receive inputs from brain structures critical for stress.
(C) Mechanical sensitivity in mice restrained 2 hr daily for 2 weeks and in unstressed littermate controls. Acute stress induced analgesia, whereas chronic stress increased mechanical sensitivity (two-way ANOVA (F(9, 144) = 4.525; * = p < 0.05).
(D) Stress-induced analgesia can be reversed by intrathecal injection of 5 μg naloxone (one-way ANOVA *p < 0.05).
(E and G) Coimmunostaining of c-Fos (green) and TLX3 (blue) PenkCre;Rosa26-LSL-tdTomato mice (red) before and after acute (E) or chronic (G) stress.
(F) Acute stress-induced analgesia was accompanied by an increase in the number of c-Fos+ Penk+ neurons that do not express TLX3 (presumably GABAergic). The total number of c-Fos+ Penk+ neurons is similar in both conditions.
(H) Chronic stress-induced hyperalgesia was accompanied by a decrease in the number of c-Fos+ Penk+ neurons not expressing TLX3 (blue) without affecting the overall population of c-Fos+ Penk+ neurons (Mann-Whitney test * = p < 0.05; *** = p < 0.0001).
PH, posterior hypothalamus; DMH, dorsomedial hypothalamus; LPB, lateral parabrachial nu; MPB, medial parabrachial nu; scp, superior cerebellar peduncle; LDTg, laterodorsal tegmental nu ventral part; CnF, cuneiform nu.
Scale bars represent 20 μm. All bar graphs represent mean ± SEM.
See also Figure S8.
