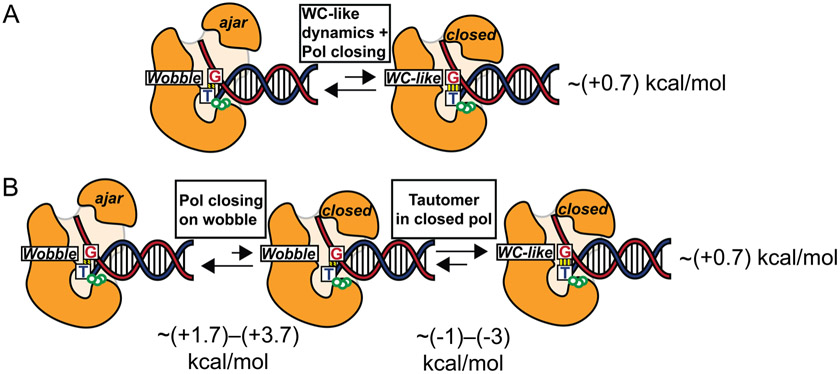
Figure 8.
(A) Equilibrium between DNA polymerase in an ajar conformation with a wobble G-T mispair and DNA polymerase in a closed conformation with a WC-like G-T mispair. A value of ~(+0.7) kcal/mol is deduced based on kinetic studies of misincorporation of DNA polyemrase β at 37 °C.7 (B) Decomposition of the equilibrium process in A into two steps involving closing of the polymerase on a wobble G-T mispair and subsequent conversion of the wobble mispair into a WC-like conformation within the closed polymerase, as computed in this work. The overall change in free energy is independent of path and therefore is the same for the paths depicted in parts A and B, allowing the deduction of the free energy associated with the first step in part B.