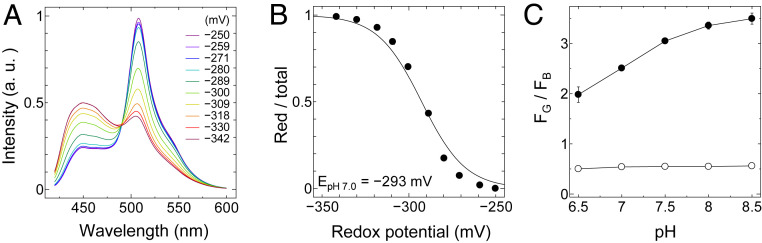
Fig. 2.
Redox potential and pH dependence of FROG/B. (A) Emission spectra of FROG/B in DTT buffers having various redox potentials. The proteins were excited at 400 nm. (B) The reduction levels of FROG/B were quantified as the ratios of the reduced forms to the total proteins and plotted against the calculated redox potentials of DTT buffers (pH 7.0). Data were fitted to the Nernst equation for two electrons exchanged: y = 1/{1 + exp[0.078 (x − Em)]}. (C) The pH dependence of the fluorescence signal ratio (FG/FB) of recombinant FROG/B. FG/FB was calculated from the fluorescence signals from their oxidized form (closed circle) and reduced form (open circle) in 100 mM Tris⋅HCl buffer with various pH levels (pH 6.5 to 8.5). For oxidation, the protein sample was treated with 20 mM DTTox overnight, and for reduction it was incubated with 10 mM DTTred.