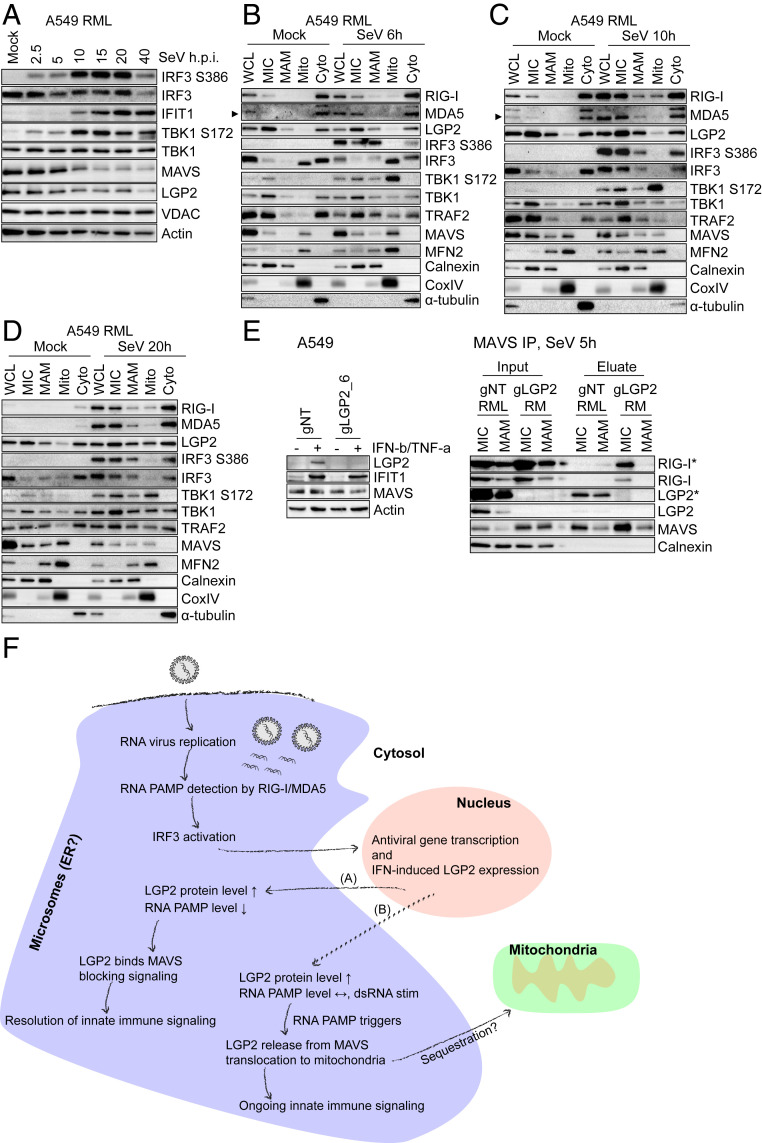
Fig. 6.
IRF3 activation does not take place on mitochondria but occurs at the ER-derived membrane network. (A) Innate immune signaling kinetics in A549-RML cells upon SeV infection. A549-RML cells were infected with 40 HAU/mL SeV and harvested for Western blot analysis at the indicated time points. Shown is one representative Western blot derived from two independent experiments (n = 2). (B–D) Subcellular fractionation of A549-RML cells after 6 h (B), 10 h (C), and 20 h (D) of SeV infection. Six independent fractionations were conducted for this experiment, comprising two fractionations per time point. (E, Left) CRISPR/Cas9-mediated LGP2 knockout in A549 cells. A549 cells were transduced with gNT or gLGP2_6 and treated with 500 IU/mL IFN-β + 10 ng/mL TNF-α 48 h prior to Western blot analysis to monitor LGP2 protein expression. (E, Right) MAVS immunoprecipitation from MIC and MAM fractions after subcellular fractionation of A549 gNT RML and A549 gLGP2 RM cells infected with 40 HAU/mL SeV for 5 h. Depicted is one representative out of two independent experiments (n = 2). Asterisks (*) indicate longer exposure. (F) Hypothetical model of RLR-mediated innate immune signaling. Upon RNA virus infection and RNA replication in viral replication compartments that are often generated at the ER, viral RNA PAMPs are detected by RIG-I/MDA5. This leads to interaction with MAVS at ER-derived membranes and IRF3 activation resulting in antiviral gene transcription, inhibition of virus replication, and IFN-induced up-regulation of LGP2 protein expression. In the case of viral PAMP reduction (A), LGP2 binds to MAVS, blocking an interaction of MAVS and RIG-I supporting the resolution of innate immune signaling. In the case of viral persistence or resistance (B), high viral RNA levels trigger LGP2 release from MAVS and its translocation to mitochondria, possibly to sequester LGP2 and allow ongoing innate immune signaling. Arrowheads point to a specific protein band.