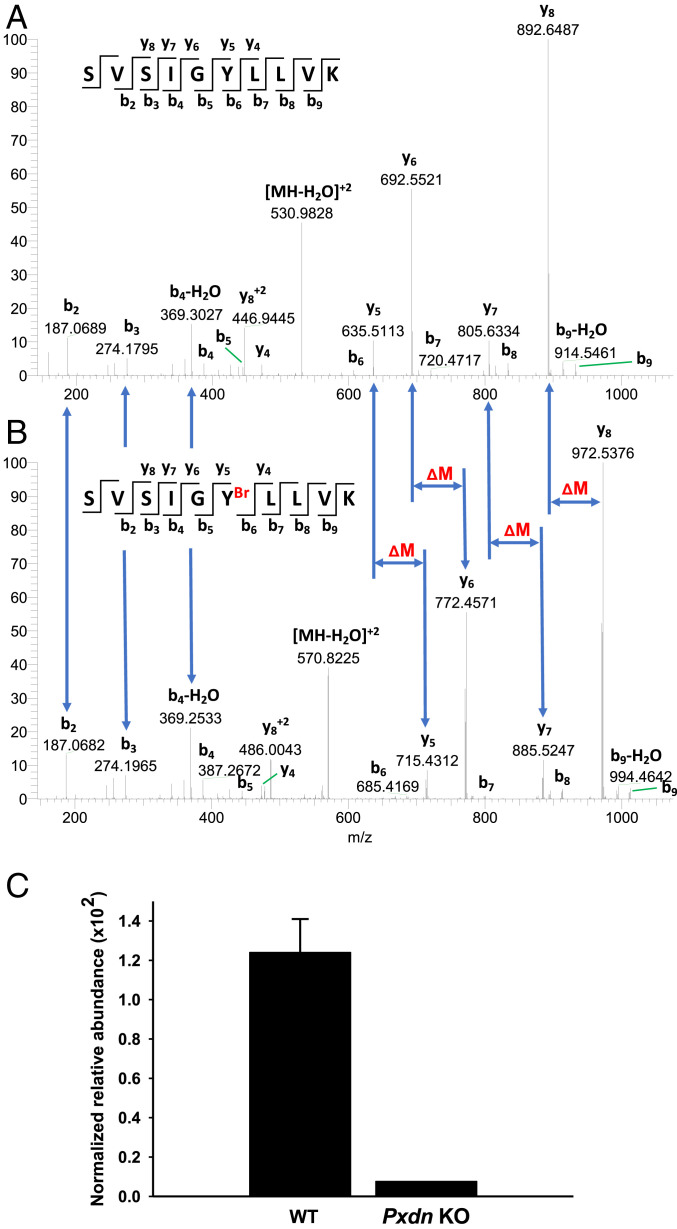
Fig. 8.
Bromination site in the α2NC1 domain of collagen IV from mouse kidney and its dependence on peroxidasin expression. NC1 domains of collagen IV were isolated from WT and Pxdn−/− mice and analyzed by LC-MS/MS as described in Materials and Methods. (A and B) Evidence for bromination of Tyr1485 in a tryptic peptide of the α2NC1 domain of mouse collagen IV [α2(IV)NC1]. Shown are MS2 spectra of unmodified (A) and brominated (B) peptide from the α2NC1 domain, including peak assignments. The mass shifts (ΔM) in y-ion series starting from y5 and indicating the sequence position of brominated tyrosine residue are also shown. Masses of 79Br and 81Br isotopes calculated from the data in A and B were 78.9107 ± 0.0271 and 80.9086 ± 0.0143, respectively. The standard isotopic masses for 79Br and 81Br are 78.9183 and 80.9163, respectively. (Insets) The sequence of unmodified (A) and brominated (B) precursor peptide and the MS2 fragmentation pattern producing specific y- and b-ions. The y axis in A and B is relative abundance. (C) Quantitation of the same brominated peptide in α2(IV)NC1 preparations from WT and Pxdn−/− mice. Normalized relative abundance represents modified peptide/(modified peptide + unmodified peptide). The WT data represent mean ± SD for three independent kidney NC1 preparations; the Pxdn−/− mouse data are from a preparation generated from the pooled kidneys of five Pxdn−/− mice.