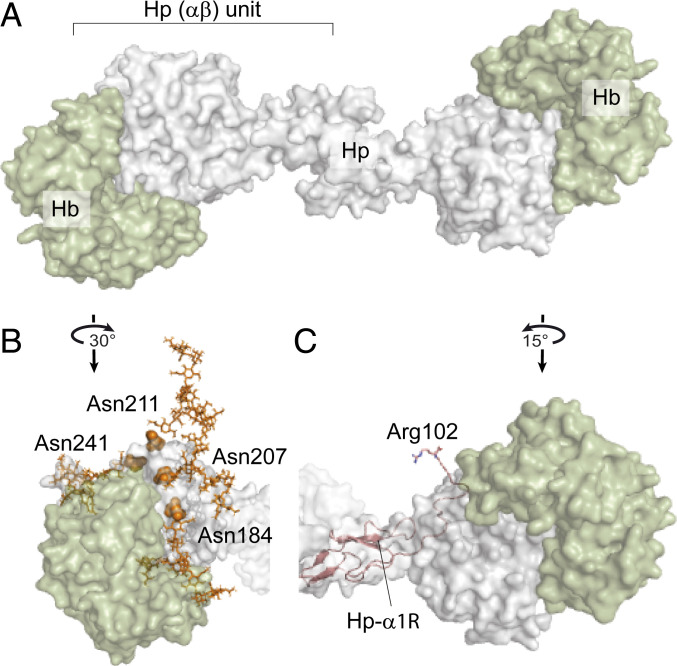
Fig. 5.
Assessing the effect of Hp glycosylation and proteolytic processing on Hp–Hb binding based on the structure of the human Hp–Hb–CD163 complex (PDB ID code 4WJG). (A) Structure of Hp 1-1 (gray) fully saturated with two Hb (αβ) dimers (green). (B) Structure of one Hp (αβ) unit bound to Hb (αβ) highlighting the Asn residues of Hp-β that carry biantennary N-glycans displayed in orange balls and sticks, respectively, revealing their proximity to the interaction site. (C) Cleaved Hp-α1R chain fitted into the available structural model of Hp 1-1 (shown as a pink cartoon) reveals that the C-terminal Arg102 is in the proximity of the Hb-α molecule (green).