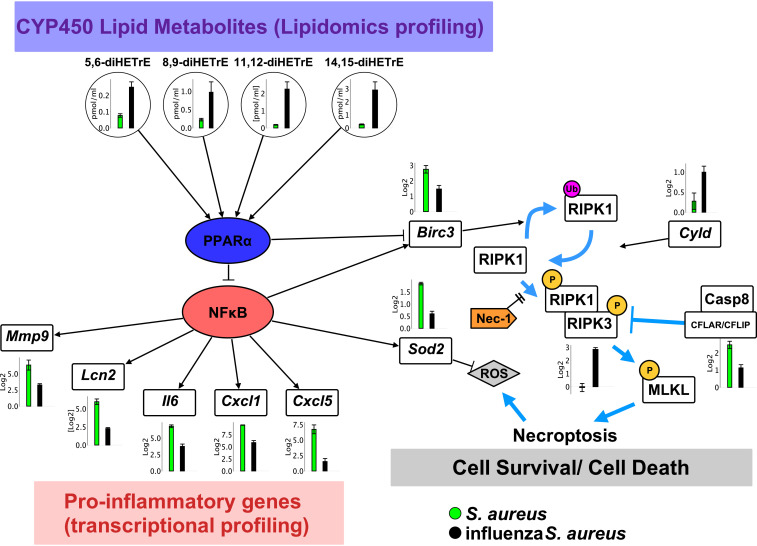
Fig. 6.
Model for the role of CYP450 metabolites in the pathophysiology of influenza/S. aureus superinfection. Levels of CYP450 lipid metabolites during influenza/S. aureus infection (black) compared to infection with S. aureus alone (green). These mediators activate the nuclear receptor PPARα, which inhibits NFκB. This inhibition is reflected in the dampened expression of numerous proinflammatory genes (Mmp9, Lcn2, Il6, Cxcl1, Cxcl5). In addition, expression of the cell survival genes Birc3 and Sod2 is also repressed in superinfected mice. Birc3 inhibits necroptosis by driving ubiquitination of RIPK1 and thereby inhibiting its phosphorylation, which is required for it to form a complex with RIPK3 and thereby drive necroptosis (37, 43). Sod2, a superoxide dismutase, clears reactive oxygen species, helping to protect against cell death under necroptotic conditions. Dampened inflammatory responses and elevated necroptosis are associated with hindered bacterial clearance and increased morbidity and mortality.